Phylogenesis of the Functional 1-Aminocyclopropane-1-Carboxylate Oxidase of Fungi and Plants
- PMID: 36675876
- PMCID: PMC9866368
- DOI: 10.3390/jof9010055
Phylogenesis of the Functional 1-Aminocyclopropane-1-Carboxylate Oxidase of Fungi and Plants
Abstract
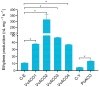
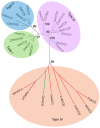
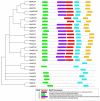
The 1-aminocyclopropane-1-carboxylic acid (ACC) pathway that synthesizes ethylene is shared in seed plants, fungi and probably other organisms. However, the evolutionary relationship of the key enzyme ACC oxidase (ACO) in the pathway among organisms remains unknown. Herein, we cloned, expressed and characterized five ACOs from the straw mushroom (Volvariella volvacea) and the oyster mushroom (Pleurotus ostreatus): VvACO1-4 and PoACO. The five mushroom ACOs and the previously identified AbACO of the button mushroom contained all three conserved residues that bound to Fe(II) in plant ACOs. They also had variable residues that were conserved and bound to ascorbate and bicarbonate in plant ACOs and harbored only 1-2 of the five conserved ACO motifs in plant ACOs. Particularly, VvACO2 and AbACO had only one ACO motif 2. Additionally, VvACO4 shared 44.23% sequence identity with the cyanobacterium Hapalosiphon putative functional ACO. Phylogenetic analysis showed that the functional ACOs of monocotyledonous and dicotyledonous plants co-occurred in Type I, Type II and Type III, while putative functional gymnosperm ACOs also appeared in Type III. The putative functional bacterial ACO, functional fungi and slime mold ACOs were clustered in ancestral Type IV. These results indicate that ACO motif 2, ACC and Fe(II) are essential for ACO activity. The ACOs of the other organisms may come from the horizontal transfer of fungal ACOs, which were found ordinarily in basidiomycetes. It is mostly the first case for the horizontal gene transfers from fungi to seed plants. The horizontal transfer of ACOs from fungi to plants probably facilitates the fungal-plant symbioses, plant-land colonization and further evolution to form seeds.
Keywords: 1-aminocyclopropane-1-carboxylic acid oxidase; basidiomycete; horizontal gene transfer; sequence motif analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Identification and active site analysis of the 1-aminocyclopropane-1-carboxylic acid oxidase catalysing the synthesis of ethylene in Agaricus bisporus.Biochim Biophys Acta. 2014 Jan;1840(1):120-8. doi: 10.1016/j.bbagen.2013.08.030. Epub 2013 Sep 6. Biochim Biophys Acta. 2014. PMID: 24016603
-
Pear ACO genes encoding putative 1-aminocyclopropane-1-carboxylate oxidase homologs are functionally expressed during fruit ripening and involved in response to salicylic acid.Mol Biol Rep. 2012 Oct;39(10):9509-19. doi: 10.1007/s11033-012-1815-5. Epub 2012 Jun 19. Mol Biol Rep. 2012. PMID: 22711312
-
1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene.Front Plant Sci. 2019 May 29;10:695. doi: 10.3389/fpls.2019.00695. eCollection 2019. Front Plant Sci. 2019. PMID: 31191592 Free PMC article. Review.
-
A novel phylogeny and morphological reconstruction of the PIN genes and first phylogeny of the ACC-oxidases (ACOs).Front Plant Sci. 2014 Jun 24;5:296. doi: 10.3389/fpls.2014.00296. eCollection 2014. Front Plant Sci. 2014. PMID: 25018760 Free PMC article.
-
1-Aminocyclopropane 1-Carboxylic Acid and Its Emerging Role as an Ethylene-Independent Growth Regulator.Front Plant Sci. 2019 Dec 5;10:1602. doi: 10.3389/fpls.2019.01602. eCollection 2019. Front Plant Sci. 2019. PMID: 31921251 Free PMC article. Review.
Cited by
-
Biotechnology of Edible Fungi.J Fungi (Basel). 2023 Oct 19;9(10):1025. doi: 10.3390/jof9101025. J Fungi (Basel). 2023. PMID: 37888281 Free PMC article.
-
Evolution of ethylene as an abiotic stress hormone in streptophytes.Environ Exp Bot. 2023 Oct;214:105456. doi: 10.1016/j.envexpbot.2023.105456. Environ Exp Bot. 2023. PMID: 37780400 Free PMC article. Review.
-
Biological formation of ethylene.RSC Chem Biol. 2023 Jul 10;4(9):635-646. doi: 10.1039/d3cb00066d. eCollection 2023 Aug 30. RSC Chem Biol. 2023. PMID: 37654506 Free PMC article. Review.
-
Functional mechanism study of the allelochemical myrigalone A identifies a group of ultrapotent inhibitors of ethylene biosynthesis in plants.Plant Commun. 2024 Jun 10;5(6):100846. doi: 10.1016/j.xplc.2024.100846. Epub 2024 Mar 8. Plant Commun. 2024. PMID: 38460510 Free PMC article.
-
H2O2 drives the transition from conchocelis to conchosporangia in the red alga Pyropia haitanensis with promotion facilitated by 1-Aminocyclopropane-1-carboxylic acid.Front Plant Sci. 2024 Mar 12;15:1379428. doi: 10.3389/fpls.2024.1379428. eCollection 2024. Front Plant Sci. 2024. PMID: 38533401 Free PMC article.
References
-
- Poel B.V.D., Cooper E.D., Delwiche C.F., Chang C. An evolutionary perspective on the plant hormone ethylene. In: Wen C.K., editor. Ethylene in Plants. Springer; Dordrecht, The Netherlands: 2015. pp. 109–134.
-
- Kende H. Ethylene biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993;44:283–307. doi: 10.1146/annurev.pp.44.060193.001435. - DOI
-
- Fukuda H., Ogawa T., Ishihara K., Fujii T., Nagahama K., Omata T., Inoue Y., Tanase S., Morino Y. Molecular cloning in Escherichia coli, expression, and nucleotide sequence of the gene for the ethylene-forming enzyme of Pseudomonas syringae pv. phaseolicola PK2. Biochem. Biophys. Res. Commun. 1992;188:826–832. doi: 10.1016/0006-291X(92)91131-9. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

