Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches
- PMID: 36675938
- PMCID: PMC9866793
- DOI: 10.3390/jof9010116
Lichen Depsides and Tridepsides: Progress in Pharmacological Approaches
Abstract
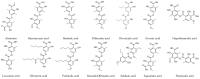
Depsides and tridepsides are secondary metabolites found in lichens. In the last 10 years, there has been a growing interest in the pharmacological activity of these compounds. This review aims to discuss the research findings related to the biological effects and mechanisms of action of lichen depsides and tridepsides. The most studied compound is atranorin, followed by gyrophoric acid, diffractaic acid, and lecanoric acid. Antioxidant, cytotoxic, and antimicrobial activities are among the most investigated activities, mainly in in vitro studies, with occasional in silico and in vivo studies. Clinical trials have not been conducted using depsides and tridepsides. Therefore, future research should focus on conducting more in vivo work and clinical trials, as well as on evaluating the other activities. Moreover, despite the significant increase in research work on the pharmacology of depsides and tridepsides, there are many of these compounds which have yet to be investigated (e.g., hiascic acid, lassalic acid, ovoic acid, crustinic acid, and hypothamnolic acid).
Keywords: depsides; lichens; pharmacological activities; tridepsides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Nugraha A.S., Untari L.F., Laub A., Porzel A., Franke K., Wessjohann L.A. Anthelmintic and antimicrobial activities of three new depsides and ten known depsides and phenols from Indonesian lichen: Parmelia cetrata Ach. Nat. Prod. Res. 2020;35:5001–5010. doi: 10.1080/14786419.2020.1761361. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources