Human Heart Morphogenesis: A New Vision Based on In Vivo Labeling and Cell Tracking
- PMID: 36676114
- PMCID: PMC9861877
- DOI: 10.3390/life13010165
Human Heart Morphogenesis: A New Vision Based on In Vivo Labeling and Cell Tracking
Abstract
Despite the extensive information available on the different genetic, epigenetic, and molecular features of cardiogenesis, the origin of congenital heart defects remains unknown. Most genetic and molecular studies have been conducted outside the context of the progressive anatomical and histological changes in the embryonic heart, which is one of the reasons for the limited knowledge of the origins of congenital heart diseases. We integrated the findings of descriptive studies on human embryos and experimental studies on chick, rat, and mouse embryos. This research is based on the new dynamic concept of heart development and the existence of two heart fields. The first field corresponds to the straight heart tube, into which splanchnic mesodermal cells from the second heart field are gradually recruited. The overall aim was to create a new vision for the analysis, diagnosis, and regionalized classification of congenital defects of the heart and great arteries. In addition to highlighting the importance of genetic factors in the development of congenital heart disease, this study provides new insights into the composition of the straight heart tube, the processes of twisting and folding, and the fate of the conus in the development of the right ventricle and its outflow tract. The new vision, based on in vivo labeling and cell tracking and enhanced by models such as gastruloids and organoids, has contributed to a better understanding of important errors in cardiac morphogenesis, which may lead to several congenital heart diseases.
Keywords: cardiogenesis; cell tracking; embryo; heart morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

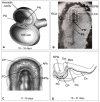
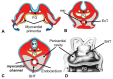

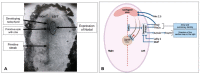
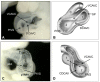
Similar articles
-
Normal development of the heart: a review of new findings.Bol Med Hosp Infant Mex. 2023;80(2):79-93. doi: 10.24875/BMHIM.22000138. Bol Med Hosp Infant Mex. 2023. PMID: 37155719 Review. English.
-
Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process.Anat Rec. 2000 Jul 1;259(3):248-62. doi: 10.1002/1097-0185(20000701)259:3<248::AID-AR30>3.0.CO;2-K. Anat Rec. 2000. PMID: 10861359 Review.
-
Incorporation of the first and second heart fields and prospective fate of the straight heart tube via in vivo labeling of chicken embryos.PLoS One. 2020 Jul 10;15(7):e0234069. doi: 10.1371/journal.pone.0234069. eCollection 2020. PLoS One. 2020. PMID: 32649674 Free PMC article.
-
The outflow tract of the heart is recruited from a novel heart-forming field.Dev Biol. 2001 Oct 1;238(1):97-109. doi: 10.1006/dbio.2001.0409. Dev Biol. 2001. PMID: 11783996
-
Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia.Dev Biol. 2005 Aug 1;284(1):72-83. doi: 10.1016/j.ydbio.2005.05.003. Dev Biol. 2005. PMID: 15950213
Cited by
-
Embryonic Hyperglycemia Disrupts Myocardial Growth, Morphological Development, and Cellular Organization: An In Vivo Experimental Study.Life (Basel). 2023 Mar 13;13(3):768. doi: 10.3390/life13030768. Life (Basel). 2023. PMID: 36983924 Free PMC article.
-
Nkx2.5: a crucial regulator of cardiac development, regeneration and diseases.Front Cardiovasc Med. 2023 Dec 6;10:1270951. doi: 10.3389/fcvm.2023.1270951. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 38124890 Free PMC article. Review.
-
Cardiac Development and Factors Influencing the Development of Congenital Heart Defects (CHDs): Part I.Int J Mol Sci. 2024 Jun 28;25(13):7117. doi: 10.3390/ijms25137117. Int J Mol Sci. 2024. PMID: 39000221 Free PMC article. Review.
-
Medical-Surgical Implications of Branching Variation of Human Aortic Arch Known as Bovine Aortic Arch (BAA).J Pers Med. 2024 Jun 24;14(7):678. doi: 10.3390/jpm14070678. J Pers Med. 2024. PMID: 39063932 Free PMC article. Review.
-
Emerging Roles of Cullin-RING Ubiquitin Ligases in Cardiac Development.Cells. 2024 Jan 26;13(3):235. doi: 10.3390/cells13030235. Cells. 2024. PMID: 38334627 Free PMC article. Review.
References
-
- Moreno-Rodriguez R.A., Krug E.L. Charlene à McQueen, Comprehensive Toxicology. Volume 6. Academic Press; Oxford, UK: 2010. Cardiovascular development; pp. 3–33.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

