A Prospective Approach to Integration of AI Fracture Detection Software in Radiographs into Clinical Workflow
- PMID: 36676172
- PMCID: PMC9864518
- DOI: 10.3390/life13010223
A Prospective Approach to Integration of AI Fracture Detection Software in Radiographs into Clinical Workflow
Abstract
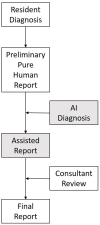
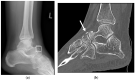
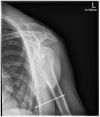
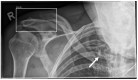
Gleamer BoneView© is a commercially available AI algorithm for fracture detection in radiographs. We aim to test if the algorithm can assist in better sensitivity and specificity for fracture detection by residents with prospective integration into clinical workflow. Radiographs with inquiry for fracture initially reviewed by two residents were randomly assigned and included. A preliminary diagnosis of a possible fracture was made. Thereafter, the AI decision on presence and location of possible fractures was shown and changes to diagnosis could be made. Final diagnosis of fracture was made by a board-certified radiologist with over eight years of experience, or if available, cross-sectional imaging. Sensitivity and specificity of the human report, AI diagnosis, and assisted report were calculated in comparison to the final expert diagnosis. 1163 exams in 735 patients were included, with a total of 367 fractures (31.56%). Pure human sensitivity was 84.74%, and AI sensitivity was 86.92%. Thirty-five changes were made after showing AI results, 33 of which resulted in the correct diagnosis, resulting in 25 additionally found fractures. This resulted in a sensitivity of 91.28% for the assisted report. Specificity was 97.11, 84.67, and 97.36%, respectively. AI assistance showed an increase in sensitivity for both residents, without a loss of specificity.
Keywords: artificial intelligence; computer-aided diagnosis; fracture; radiographs.
Conflict of interest statement
Stefan Niehues has received research grants from Bracco Group, Bayer Vital GmbH, Canon Medical Systems, and Guerbet. Bernd Hamm has received research grants for the Department of Radiology, Charité—Universitätsmedizin Berlin from the following companies: (1) Abbott, (2) Actelion Pharmaceuticals, (3) Bayer Schering Pharma, (4) Bayer Vital, (5) BRACCO Group, (6) Bristol-Myers Squibb, (7) Charite Research Organisation GmbH, (8) Deutsche Krebshilfe, (9) Dt. Stiftung für Herzforschung, (10) Essex Pharma, (11) EU Programmes, (12) FibrexMedical Inc, (13) Focused Ultrasound Surgery Foundation, (14) Fraunhofer Gesellschaft, (15) Guerbet, (16) INC Research, (17) lnSightec Ud, (18) IPSEN Pharma, (19) Kendlel MorphoSys AG, (20) Lilly GmbH, (21) Lundbeck GmbH, (22) MeVis Medical Solutions AG, (23) Nexus Oncology, (24) Novartis, (25) Parexel Clinical Research Organisation Service, (26) Perceptive, (27) Pfizer GmbH, (28) Philipps, (29) Sanofis-Aventis S.A., (30) Siemens, (31) Spectranetics GmbH, (32) Terumo Medical Corporation, (33) TNS Healthcare GMbH, (34) Toshiba, (35) UCB Pharma, (36) Wyeth Pharma, (37) Zukunftsfond Berlin (TSB), (38) Amgen, (39) AO Foundation, (40) BARD, (41) BBraun, (42) Boehring Ingelheimer, (43) Brainsgate, (44) PPD (Clinical Research Organisation), (45) CELLACT Pharma, (46) Celgene, (47) CeloNova Bio-Sciences, (48) Covance, (49) DC Deviees, Ine. USA, (50) Ganymed, (51) Gilead Sciences, (52) GlaxoSmithKline, (53) ICON (Clinical Research Organisation), (54) Jansen, (55) LUX Bioseienees, (56) MedPass, (57) Merek, (58) Mologen, (59) Nuvisan, (60) Pluristem, (61) Quintiles, (62) Roehe, (63) SehumaeherGmbH (Sponsoring eines Workshops), (64) Seattle Geneties, (65) Symphogen, (66) TauRx Therapeuties Ud, (67) Accovion, (68) AIO: Arbeitsgemeinschaft Internistische Onkologie, (69) ASR Advanced sleep research, (70) Astellas, (71) Theradex, (72) Galena Biopharma, (73) Chiltern, (74) PRAint, (75) lnspiremd, (76) Medronic, (77) Respicardia, (78) Silena Therapeutics, (79) Spectrum Pharmaceuticals, (80) St Jude, (81) TEVA, (82) Theorem, (83) Abbvie, (84) Aesculap, (85) Biotronik, (86) Inventivhealth, (87) ISATherapeutics, (88) LYSARC, (89) MSD, (90) Novocure, (91) Ockham Oncology, (92) Premier-Research, (93) Psi-cro, (94) Tetec-ag, (95) Winicker-Norimed, (96) Achaogen Inc, (97) ADIR, (98) AstraZenaca AB, (99) Demira Inc, (100) Euroscreen S.A., (101) Galmed Research and Development Ltd., (102) GETNE, (103) Guidant Europe NV, (104) Holaira Inc, (105) Immunomedics Inc, (106) Innate Pharma, (107) Isis Pharmaceuticals Inc, (108) Kantar Health GmbH, (109) MedImmune Inc, (110) Medpace Germany GmbH (CRO), (111) Merrimack Pharmaceuticals Inc, (112) Millenium Pharmaceuticals Inc, (113) Orion Corporation Orion Pharma, (114) Pharmacyclics Inc, (115) PIQUR Therapeutics Ltd., (116) Pulmonx International Sárl, (117) Servier (CRO), (118) SGS Life Science Services (CRO), and (119) Treshold Pharmaceuticals Inc. These grants had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that they have no conflicts of interest and did not receive any funds.
Figures

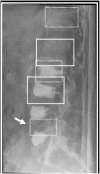
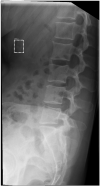
References
-
- Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. [(accessed on 21 October 2022)]; Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artific....
-
- Guermazi A., Tannoury C., Kompel A.J., Murakami A.M., Ducarouge A., Gillibert A., Li X., Tournier A., Lahoud Y., Jarraya M., et al. Improving Radiographic Fracture Recognition Performance and Efficiency Using Artificial Intelligence. Radiology. 2021;302:627–636. doi: 10.1148/radiol.210937. - DOI - PubMed
-
- Duron L., Ducarouge A., Gillibert A., Laine J., Allouche C., Cherel N., Zhang Z., Nitche N., Lacave E., Pourchot A., et al. Assessment of an AI Aid in Detection of Adult Appendicular Skeletal Fractures by Emergency Physicians and Radiologists: A Multicenter Cross-sectional Diagnostic Study. Radiology. 2021;300:120–129. doi: 10.1148/radiol.2021203886. - DOI - PubMed
LinkOut - more resources
Full Text Sources

