Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)?
- PMID: 36676175
- PMCID: PMC9863729
- DOI: 10.3390/life13010226
Targeting Neuroinflammation to Alleviate Chronic Olfactory Dysfunction in Long COVID: A Role for Investigating Disease-Modifying Therapy (DMT)?
Abstract
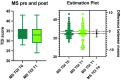
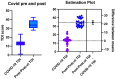
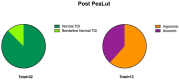
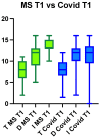
Chronic olfactory dysfunction after SARS-CoV-2 infection occurs in approximately 10% of patients with COVID-19-induced anosmia, and it is a growing public health concern. A regimen of olfactory training and anti-neuroinflammatory therapy with co-ultramicronized palmitoylethanolamide with luteolin (um-PEA-LUT) has shown promising results in clinical trials; however, approximately 15% of treated patients do not achieve full recovery of a normal olfactory threshold, and almost 5% have no recovery. Disease-modifying therapies (DMTs), which are used to treat autoimmune neuroinflammation in multiple sclerosis (MS), have not been studied for treating persistent inflammation in refractory post-COVID-19 smell disorder. This study evaluated COVID-19-related smell loss and MS-related smell loss, comparing the responses to different therapies. Forty patients with MS and 45 reporting post-COVID-19 olfactory disorders were included in the study. All patients underwent nasal endoscopy and were evaluated by using validated Sniffin' Sticks testing. The patients with long COVID were treated for three months with um-PEA-LUT plus olfactory training. The patients with MS were treated with DMTs. Olfactory functions before and after treatment were analyzed in both groups. At the experimental endpoint, 13 patients in the COVID-19 group treated with um-PEA-LUT had residual olfactory impairment versus 10 patients in the MS group treated with DMTs. The severity of the persistent olfactory loss was lower in the MS group, and the patients with MS treated with IFN-beta and glatiramer acetate had the preservation of olfactory function. These data provide a rationale for considering prospective trials investigating the efficacy of DMTs for post-COVID-19 olfactory disorders that are refractory to um-PEA-LUT with olfactory training. This study is the first to consider the role of DMT in treating refractory post-viral olfactory loss in patients with long COVID.
Keywords: COVID-19; PASC; SARS-CoV-2; TDM; anosmia; disease-modifying therapy; hyposmia; interferon; long COVID; long-haul COVID; multiple sclerosis; neuroinflammation; olfaction; olfactory; olfactory training; post-acute sequelae of SARS-CoV-2 infection; smell; smell disorders; threshold detection identification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Treatment of COVID-19 olfactory dysfunction with olfactory training, palmitoylethanolamide with luteolin, or combined therapy: a blinded controlled multicenter randomized trial.Eur Arch Otorhinolaryngol. 2023 Nov;280(11):4949-4961. doi: 10.1007/s00405-023-08085-8. Epub 2023 Jun 28. Eur Arch Otorhinolaryngol. 2023. PMID: 37380908 Free PMC article. Clinical Trial.
-
Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo- Controlled Clinical Trial.Curr Neuropharmacol. 2022;20(10):2001-2012. doi: 10.2174/1570159X20666220420113513. Curr Neuropharmacol. 2022. PMID: 35450527 Free PMC article. Clinical Trial.
-
Effect of Ultra-Micronized Palmitoylethanolamide and Luteolin on Olfaction and Memory in Patients with Long COVID: Results of a Longitudinal Study.Cells. 2022 Aug 17;11(16):2552. doi: 10.3390/cells11162552. Cells. 2022. PMID: 36010630 Free PMC article. Clinical Trial.
-
Effect of any form of steroids in comparison with that of other medications on the duration of olfactory dysfunction in patients with COVID-19: A systematic review of randomized trials and quasi-experimental studies.PLoS One. 2023 Aug 2;18(8):e0288285. doi: 10.1371/journal.pone.0288285. eCollection 2023. PLoS One. 2023. PMID: 37531338 Free PMC article.
-
Risk of COVID-19 infection and severe disease in MS patients on different disease-modifying therapies.Mult Scler Relat Disord. 2022 Apr;60:103735. doi: 10.1016/j.msard.2022.103735. Epub 2022 Mar 11. Mult Scler Relat Disord. 2022. PMID: 35398713 Free PMC article.
Cited by
-
Post-COVID Condition and Neuroinflammation: Possible Management with Antioxidants.Antioxidants (Basel). 2025 Jul 8;14(7):840. doi: 10.3390/antiox14070840. Antioxidants (Basel). 2025. PMID: 40722944 Free PMC article. Review.
-
Persistent Post-COVID-19 Olfactory Dysfunction and Its Association with Autonomic Nervous System Function: A Case-Control Study.Diseases. 2024 Dec 28;13(1):4. doi: 10.3390/diseases13010004. Diseases. 2024. PMID: 39851468 Free PMC article.
-
Treatments for Olfactory Dysfunction in COVID-19: A Systematic Review.Int Arch Otorhinolaryngol. 2024 May 25;28(4):e728-e743. doi: 10.1055/s-0044-1786046. eCollection 2024 Oct. Int Arch Otorhinolaryngol. 2024. PMID: 39464360 Free PMC article. Review.
-
Receptors Involved in COVID-19-Related Anosmia: An Update on the Pathophysiology and the Mechanistic Aspects.Int J Mol Sci. 2024 Aug 5;25(15):8527. doi: 10.3390/ijms25158527. Int J Mol Sci. 2024. PMID: 39126095 Free PMC article. Review.
-
Possible Role of Cannabis in the Management of Neuroinflammation in Patients with Post-COVID Condition.Int J Mol Sci. 2024 Mar 29;25(7):3805. doi: 10.3390/ijms25073805. Int J Mol Sci. 2024. PMID: 38612615 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

