The New Materials for Battery Electrode Prototypes
- PMID: 36676291
- PMCID: PMC9862395
- DOI: 10.3390/ma16020555
The New Materials for Battery Electrode Prototypes
Abstract
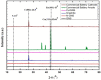
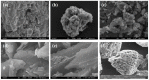
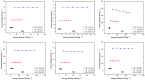
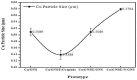
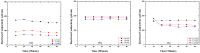
In this article, we present the performance of Copper (Cu)/Graphene Nano Sheets (GNS) and C-π (Graphite, GNS, and Nitrogen-doped Graphene Nano Sheets (N-GNS)) as a new battery electrode prototype. The objectives of this research are to develop a number of prototypes of the battery electrode, namely Cu/GNS//Electrolyte//C-π, and to evaluate their respective performances. The GNS, N-GNS, and primary battery electrode prototypes (Cu/GNS/Electrolyte/C-π) were synthesized by using a modified Hummers method; the N-doped sheet was obtained by doping nitrogen at room temperature and the impregnation or the composite techniques, respectively. Commercial primary battery electrodes were also used as a reference in this research. The Graphite, GNS, N-GNS, commercial primary batteries electrode, and battery electrode prototypes were analyzed using an XRD, SEM-EDX, and electrical multimeter, respectively. The research data show that the Cu particles are well deposited on the GNS and N-GNS (XRD and SEM-EDX data). The presence of the Cu metal and electrolytes (NH4Cl and MnO2) materials can increase the electrical conductivities (335.6 S cm-1) and power density versus the energy density (4640.47 W kg-1 and 2557.55 Wh kg-1) of the Cu/GNS//Electrolyte//N-GNS compared to the commercial battery (electrical conductivity (902.2 S cm-1) and power density versus the energy density (76 W kg-1 and 43.95 W kg-1). Based on all of the research data, it may be concluded that Cu/GNS//Electrolyte//N-GNS can be used as a new battery electrode prototype with better performances and electrical activities.
Keywords: Cu/GNS; N-graphene nano sheets; battery electrode prototype; electrolyte; graphene nano sheets; graphite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Chen J., Yao B., Li C., Shi G. An Improved Hummers Method for Eco-Friendly Synthesis of Graphene Oxide. Carbon. 2013;64:225–229. doi: 10.1016/j.carbon.2013.07.055. - DOI
-
- Zhu S., Zhang F., Lu H.G., Sheng J., Wang L., Li S.D., Han G., Li Y. Flash Nitrogen-Doped Graphene for High-Rate Supercapacitors. ACS Mater. Lett. 2022;4:1863–1871. doi: 10.1021/acsmaterialslett.2c00616. - DOI
-
- Siburian R., Paiman S., Hutagalung F., Ali A.M.M., Simatupang L., Goei R., Rusop M.M. Facile method to synthesize of magnesium-graphene nano sheets for candidate of primary battery electrode. Colloids Interface Sci. Commun. 2022;48:100612. doi: 10.1016/j.colcom.2022.100612. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

