State-of-the-Art of Polymer/Fullerene C60 Nanocomposite Membranes for Water Treatment: Conceptions, Structural Diversity and Topographies
- PMID: 36676834
- PMCID: PMC9864887
- DOI: 10.3390/membranes13010027
State-of-the-Art of Polymer/Fullerene C60 Nanocomposite Membranes for Water Treatment: Conceptions, Structural Diversity and Topographies
Abstract
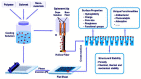
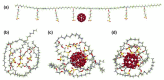
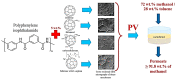
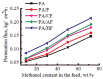
To secure existing water resources is one of the imposing challenges to attain sustainability and ecofriendly world. Subsequently, several advanced technologies have been developed for water treatment. The most successful methodology considered so far is the development of water filtration membranes for desalination, ion permeation, and microbes handling. Various types of membranes have been industrialized including nanofiltration, microfiltration, reverse osmosis, and ultrafiltration membranes. Among polymeric nanocomposites, nanocarbon (fullerene, graphene, and carbon nanotubes)-reinforced nanomaterials have gained research attention owing to notable properties/applications. Here, fullerene has gained important stance amid carbonaceous nanofillers due to zero dimensionality, high surface areas, and exceptional physical properties such as optical, electrical, thermal, mechanical, and other characteristics. Accordingly, a very important application of polymer/fullerene C60 nanocomposites has been observed in the membrane sector. This review is basically focused on talented applications of polymer/fullerene nanocomposite membranes in water treatment. The polymer/fullerene nanostructures bring about numerous revolutions in the field of high-performance membranes because of better permeation, water flux, selectivity, and separation performance. The purpose of this pioneering review is to highlight and summarize current advances in the field of water purification/treatment using polymer and fullerene-based nanocomposite membranes. Particular emphasis is placed on the development of fullerene embedded into a variety of polymer membranes (Nafion, polysulfone, polyamide, polystyrene, etc.) and effects on the enhanced properties and performance of the resulting water treatment membranes. Polymer/fullerene nanocomposite membranes have been developed using solution casting, phase inversion, electrospinning, solid phase synthesis, and other facile methods. The structural diversity of polymer/fullerene nanocomposites facilitates membrane separation processes, especially for valuable or toxic metal ions, salts, and microorganisms. Current challenges and opportunities for future research have also been discussed. Future research on these innovative membrane materials may overwhelm design and performance-related challenging factors.
Keywords: fullerene C60; membrane; nanocomposite; nanofiltration; permeability; polymer; salt rejection; selectivity; water treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Engineered Zero-Dimensional Fullerene/Carbon Dots-Polymer Based Nanocomposite Membranes for Wastewater Treatment.Molecules. 2020 Oct 26;25(21):4934. doi: 10.3390/molecules25214934. Molecules. 2020. PMID: 33114470 Free PMC article. Review.
-
Molecular modeling of thin-film nanocomposite membranes for reverse osmosis water desalination.Phys Chem Chem Phys. 2022 Dec 14;24(48):29298-29327. doi: 10.1039/d2cp03839k. Phys Chem Chem Phys. 2022. PMID: 36453147 Review.
-
Advanced Polymeric Nanocomposite Membranes for Water and Wastewater Treatment: A Comprehensive Review.Polymers (Basel). 2023 Jan 20;15(3):540. doi: 10.3390/polym15030540. Polymers (Basel). 2023. PMID: 36771842 Free PMC article. Review.
-
A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification.Polymers (Basel). 2019 Jul 29;11(8):1252. doi: 10.3390/polym11081252. Polymers (Basel). 2019. PMID: 31362430 Free PMC article. Review.
-
Copper-Modified Polymeric Membranes for Water Treatment: A Comprehensive Review.Membranes (Basel). 2021 Jan 28;11(2):93. doi: 10.3390/membranes11020093. Membranes (Basel). 2021. PMID: 33525631 Free PMC article. Review.
Cited by
-
Hydrophilization and Functionalization of Fullerene C60 with Maleic Acid Copolymers by Forming a Non-Covalent Complex.Polymers (Basel). 2024 Jun 19;16(12):1736. doi: 10.3390/polym16121736. Polymers (Basel). 2024. PMID: 38932086 Free PMC article.
References
-
- Kausar A. Graphene to Polymer/Graphene Nanocomposites. Elsevier; Amsterdam, The Netherlands: 2021. Gas separation and filtration membrane applications of polymer/graphene nanocomposites; pp. 197–222. - DOI
Publication types
LinkOut - more resources
Full Text Sources

