Lamellarity-Driven Differences in Surface Structural Features of DPPS Lipids: Spectroscopic, Calorimetric and Computational Study
- PMID: 36676890
- PMCID: PMC9865892
- DOI: 10.3390/membranes13010083
Lamellarity-Driven Differences in Surface Structural Features of DPPS Lipids: Spectroscopic, Calorimetric and Computational Study
Abstract
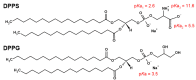
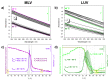
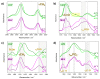
Although single-lipid bilayers are usually considered models of eukaryotic plasma membranes, their research drops drastically when it comes to exclusively anionic lipid membranes. Being a major anionic phospholipid in the inner leaflet of eukaryote membranes, phosphatidylserine-constituted lipid membranes were occasionally explored in the form of multilamellar liposomes (MLV), but their inherent instability caused a serious lack of efforts undertaken on large unilamellar liposomes (LUVs) as more realistic model membrane systems. In order to compensate the existing shortcomings, we performed a comprehensive calorimetric, spectroscopic and MD simulation study of time-varying structural features of LUV made from 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine (DPPS), whereas the corresponding MLV were examined as a reference. A substantial uncertainty of UV/Vis data of LUV from which only Tm was unambiguously determined (53.9 ± 0.8 °C), along with rather high uncertainty on the high-temperature range of DPPS melting profile obtained from DSC (≈50-59 °C), presumably reflect distinguished surface structural features in LUV. The FTIR signatures of glycerol moiety and those originated from carboxyl group serve as a strong support that in LUV, unlike in MLV, highly curved surfaces occur continuously, whereas the details on the attenuation of surface features in MLV were unraveled by molecular dynamics.
Keywords: 1,2-dipalmitoyl-sn-glycero-3-phospho-L-serine sodium salt (DPPS); MD simulations; interbilayer water; multilamellar and large unilamellar vesicles (MLV and LUV); spectroscopic and calorimetric study; surface curvature fluctuations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Deciphering the origin of the melting profile of unilamellar phosphatidylcholine liposomes by measuring the turbidity of its suspensions.Soft Matter. 2022 Sep 14;18(35):6703-6715. doi: 10.1039/d2sm00878e. Soft Matter. 2022. PMID: 36017811
-
Interaction of guanidinium and ammonium cations with phosphatidylcholine and phosphatidylserine lipid bilayers - Calorimetric, spectroscopic and molecular dynamics simulations study.Biochim Biophys Acta Biomembr. 2023 Apr;1865(4):184122. doi: 10.1016/j.bbamem.2023.184122. Epub 2023 Feb 3. Biochim Biophys Acta Biomembr. 2023. PMID: 36739930
-
Uncoated gold nanoparticles create fewer and less localized defects in model prokaryotic than in model eukaryotic lipid membranes.Colloids Surf B Biointerfaces. 2024 Nov;243:114158. doi: 10.1016/j.colsurfb.2024.114158. Epub 2024 Aug 12. Colloids Surf B Biointerfaces. 2024. PMID: 39137531
-
Influence of DPPE surface undulations on melting temperature determination: UV/Vis spectroscopic and MD study.Biochim Biophys Acta Biomembr. 2023 Jan 1;1865(1):184072. doi: 10.1016/j.bbamem.2022.184072. Epub 2022 Oct 8. Biochim Biophys Acta Biomembr. 2023. PMID: 36216096
-
The rise of FTIR spectroscopy in the characterization of asymmetric lipid membranes.Spectrochim Acta A Mol Biomol Spectrosc. 2024 Jan 15;305:123488. doi: 10.1016/j.saa.2023.123488. Epub 2023 Oct 5. Spectrochim Acta A Mol Biomol Spectrosc. 2024. PMID: 37813090
Cited by
-
Adsorption/Desorption of Cationic-Hydrophobic Peptides on Zwitterionic Lipid Bilayer Is Associated with the Possibility of Proton Transfer.Antibiotics (Basel). 2023 Jul 21;12(7):1216. doi: 10.3390/antibiotics12071216. Antibiotics (Basel). 2023. PMID: 37508312 Free PMC article.
-
Phase-Dependent Adsorption of Myelin Basic Protein to Phosphatidylcholine Lipid Bilayers.Membranes (Basel). 2024 Jan 4;14(1):15. doi: 10.3390/membranes14010015. Membranes (Basel). 2024. PMID: 38248705 Free PMC article.
-
Odor Discrimination by Lipid Membranes.Membranes (Basel). 2023 Jan 24;13(2):151. doi: 10.3390/membranes13020151. Membranes (Basel). 2023. PMID: 36837654 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

