Nanovesicles as Vanillin Carriers for Antimicrobial Applications
- PMID: 36676902
- PMCID: PMC9865702
- DOI: 10.3390/membranes13010095
Nanovesicles as Vanillin Carriers for Antimicrobial Applications
Abstract
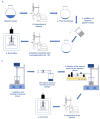
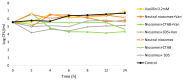
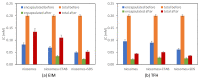
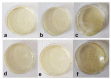
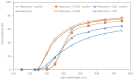
Vanillin is a natural compound easily extracted from plants. It has neuroprotective, anti-carcinogenic, antioxidant, antimicrobial, and anti-biofilm properties. It also presents high volatility, high hydrophilicity, and low bioavailability. Nanomaterials can be used to improve pharmacodynamics, solubility, and stability and to enhance pharmacokinetics. In this work, non-ionic surfactant vesicles were synthesized as vanillin carriers: neutral niosomes formed by Span60 and cholesterol, positive charged niosomes formulated with cetyltrimethylammonium bromide (CTAB), and negatively charged niosomes formulated with sodium dodecyl sulfate (SDS). Niosomes synthesis was carried out with two commonly used methods: thin film hydration (TFH) and ethanol injection method (EIM). The niosomes synthesized were used to prepare two different materials: (i) a powder containing the lyophilized noisome with vanillin systems and (ii) a gelatin matrix film containing niosomes with vanillin. Lyophilization was carried out using maltodextrin as a cryoprotectant. The lyophilization of colloidal structures allows for storage at room temperature for long periods of time, keeping their organoleptic characteristics invariable. Niosomes were characterized before and after the lyophilization process in terms of morphological characterization, size, polydispersity index (PDI), and zeta potential. Moreover, niosomes cargo was evaluated by calculating the encapsulation efficiency (EE) and loading capacity (LC). Results showed that the use of the TFH method allowed us to obtain niosomes of 255 nm with high EE (up to 40%) and LC values higher than EIM. The lyophilization process decreased the LC of the vesicles prepared, but this decrease was mitigated by up to 20% when ionic surfactants were used on the membrane bilayer. Gelatin films are biodegradable materials suitable for food packing applications. The incorporation of a natural compound with antimicrobial activity would be a clear advantage for such an application. The films prepared were characterized in terms of morphology, water solubility, color, and transparency. Niosomes synthesized by thin film hydration had better chemical and physical properties to load vanillin. Especially in the case of application in films, niosomes with a negative charge, formed by SDS, and vanillin loaded gave better mechanical and chemical characteristics to the film.
Keywords: antimicrobial properties; encapsulation; gelatin films; lyophilization; vanillin; vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Arya S.S., Rookes J.E., Cahill D.M., Lenka S.K. Vanillin: A Review on the Therapeutic Prospects of a Popular Flavouring Molecule. Adv. Tradit. Med. 2021;21:415–431. doi: 10.1007/s13596-020-00531-w. - DOI
-
- Arya S.S., Sharma M.M., Rookes J.E., Cahill D.M., Lenka S.K. Vanilla Modulates the Activity of Antibiotics and Inhibits Efflux Pumps in Drug-Resistant Pseudomonas Aeruginosa. Biologia. 2021;76:781–791. doi: 10.2478/s11756-020-00617-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

