Still Excited, but Less Aroused-The Effects of Nutritional Ketosis on Epinephrine Response and Hypothalamic Orexin Neuron Activation Following Recurrent Hypoglycemia in Diabetic Rats
- PMID: 36676967
- PMCID: PMC9862750
- DOI: 10.3390/metabo13010042
Still Excited, but Less Aroused-The Effects of Nutritional Ketosis on Epinephrine Response and Hypothalamic Orexin Neuron Activation Following Recurrent Hypoglycemia in Diabetic Rats
Abstract
Hypoglycemia-associated autonomic failure (HAAF) is a serious, life-threatening complication of intensive insulin therapy, particularly in people with type 1 diabetes. The ketogenic diet is reported to beneficially affect glycemic control in people with type 1 diabetes, however its effects on the neurohormonal counterregulatory response to recurrent hypoglycemia and HAAF development are understudied. In this study we used Sprague Dawley rats to establish a HAAF model under non-diabetic and streptozotocin (STZ)-induced diabetic conditions and determined how nutritional ketosis affected the neurohormonal counterregulation and the activity of energy-sensing orexin (OX) neurons. We found that antecedent hypoglycemia diminished the sympathoexcitatory epinephrine response to subsequent hypoglycemia in chow-fed non-diabetic rats, but this did not occur in STZ-diabetic animals. In all cases a ketogenic diet preserved the epinephrine response. Contrary to expectations, STZ-diabetic keto-fed rats showed reduced OX activity in the recurrent hypoglycemia group, which did not occur in any other group. It is possible that the reduced activation of OX neurons is an adaptation aimed at energy conservation accompanied by diminished arousal and exploratory behaviour. Our data suggests that while a ketogenic diet has beneficial effects on glycemia, and epinephrine response, the reduced activation of OX neurons could be detrimental and warrants further investigation.
Keywords: STZ-diabetes; counterregulatory response; epinephrine; insulin-induced hypoglycemia; ketogenic diet; ketosis; orexin neurons; rat model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


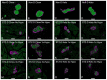

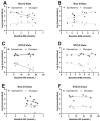

References
-
- Newburgh L.H., Marsh P.L. The use of a high fat diet in the treatment of diabetes mellitus: First paper. Arch. Intern. Med. 1920;26:647–662. doi: 10.1001/archinte.1920.00100060002001. - DOI
-
- Newburgh L.H., Marsh P.L. The use of a high fat diet in the treatment of diabetes mellitus: Second paper: Blood sugar. Arch. Intern. Med. 1921;27:699–705. doi: 10.1001/archinte.1921.00100120070005. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

