A Review on Electrochemical Microsensors for Ascorbic Acid Detection: Clinical, Pharmaceutical, and Food Safety Applications
- PMID: 36677102
- PMCID: PMC9864818
- DOI: 10.3390/mi14010041
A Review on Electrochemical Microsensors for Ascorbic Acid Detection: Clinical, Pharmaceutical, and Food Safety Applications
Abstract
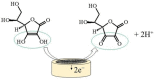
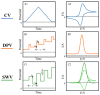
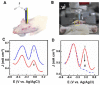
Nowadays, micro-sized sensors have become a hot topic in electroanalysis. Because of their excellent analytical features, microelectrodes are well-accepted tools for clinical, pharmaceutical, food safety, and environmental applications. In this brief review, we highlight the state-of-art electrochemical non-enzymatic microsensors for quantitative detection of ascorbic acid (also known as vitamin C). Ascorbic acid is a naturally occurring water-soluble organic compound with antioxidant properties and its quantitative determination in biological fluids, foods, cosmetics, etc., using electrochemical microsensors is of wide interest. Various electrochemical techniques have been applied to detect ascorbic acid with extremely high sensitivity, selectivity, reproducibility, and reliability, and apply to in vivo measurements. This review paper aims to give readers a clear view of advances in areas of electrode modification, successful strategies for signal amplification, and miniaturization techniques used in the electroanalytical devices for ascorbic acid. In conclusion, current challenges related to the microelectrodes design, and future perspectives are outlined.
Keywords: electroanalysis; microelectrodes; sensors; vitamin C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Devaki S.J., Raveendran R.L. Vitamin C: Sources, Functions, Sensing and Analysis. In: Hamza A., editor. Vitamin C. IntechOpen; London, UK: 2017. - DOI
-
- Vissers M.C.M., Andrew A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. [(accessed on 15 October 2022)];Front. Physiol. 2018 9:809. doi: 10.3389/fphys.2018.00809. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2018.00809. - DOI - DOI - PMC - PubMed
-
- Figueroa-Méndez R., Rivas-Arancibia S. Vitamin C in Health and Disease: Its Role in the Metabolism of Cells and Redox State in the Brain. [(accessed on 15 October 2022)];Front. Physiol. 2015 6:397. doi: 10.3389/fphys.2015.00397. Available online: https://www.frontiersin.org/articles/10.3389/fphys.2015.00397. - DOI - DOI - PMC - PubMed
-
- Steinberg F.M., Rucker R.B. Vitamin C. In: Lennarz W.J., Lane M.D., editors. Encyclopedia of Biological Chemistry. 2nd ed. Elsevier Inc.; Amsterdam, The Netherlands: 2013. pp. 530–534. - DOI
Publication types
LinkOut - more resources
Full Text Sources

