Advances in BBB on Chip and Application for Studying Reversible Opening of Blood-Brain Barrier by Sonoporation
- PMID: 36677173
- PMCID: PMC9861620
- DOI: 10.3390/mi14010112
Advances in BBB on Chip and Application for Studying Reversible Opening of Blood-Brain Barrier by Sonoporation
Abstract
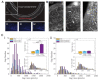
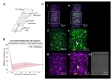
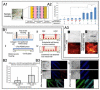
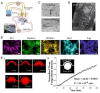
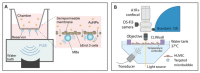
The complex structure of the blood-brain barrier (BBB), which blocks nearly all large biomolecules, hinders drug delivery to the brain and drug assessment, thus decelerating drug development. Conventional in vitro models of BBB cannot mimic some crucial features of BBB in vivo including a shear stress environment and the interaction between different types of cells. There is a great demand for a new in vitro platform of BBB that can be used for drug delivery studies. Compared with in vivo models, an in vitro platform has the merits of low cost, shorter test period, and simplicity of operation. Microfluidic technology and microfabrication are good tools in rebuilding the BBB in vitro. During the past decade, great efforts have been made to improve BBB penetration for drug delivery using biochemical or physical stimuli. In particular, compared with other drug delivery strategies, sonoporation is more attractive due to its minimized systemic exposure, high efficiency, controllability, and reversible manner. BBB on chips (BOC) holds great promise when combined with sonoporation. More details and mechanisms such as trans-endothelial electrical resistance (TEER) measurements and dynamic opening of tight junctions can be figured out when using sonoporation stimulating BOC, which will be of great benefit for drug development. Herein, we discuss the recent advances in BOC and sonoporation for BBB disruption with this in vitro platform.
Keywords: blood–brain barrier; microfluidics; ultrasound-mediated drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Alzheimer’s Association 2020 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2020;14:367–429. doi: 10.1002/alz.12068. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

