Omicron Sub-Lineage BA.5 and Recombinant XBB Evasion from Antibody Neutralisation in BNT162b2 Vaccine Recipients
- PMID: 36677483
- PMCID: PMC9866687
- DOI: 10.3390/microorganisms11010191
Omicron Sub-Lineage BA.5 and Recombinant XBB Evasion from Antibody Neutralisation in BNT162b2 Vaccine Recipients
Abstract
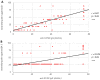
The recent emergence of a number of new SARS-CoV-2 variants resulting from recombination between two distinct parental lineages or sub-lineages within the same lineage has sparked the debate regarding potential enhanced viral infectivity and immune escape. Among these, XBB, recombinant of BA.2.10 and BA.2.75, has caused major concern in some countries due to its rapid increase in prevalence. In this study, we tested XBB escape capacity from mRNA-vaccine-induced (BNT162b2) neutralising antibodies compared to B.1 ancestral lineage and another co-circulating variant (B.1.1.529 BA.5) by analysing sera collected 30 days after the second dose in 92 healthcare workers. Our data highlighted an enhanced and statistically significant immune escape ability of the XBB recombinant. Although these are preliminary results, this study highlights the importance of immune escape monitoring of new and forthcoming variants and of the reformulation of existing vaccines.
Keywords: SARS-CoV-2; XBB recombinant; immune escape; mRNA-vaccine; neutralising antibody response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization Coronavirus Disease (COVID-2019) Dashboard. [(accessed on 30 November 2022)]. Available online: https://covid19.who.int/
-
- World Health Organization Tracking SARS-CoV-2 Variants. [(accessed on 30 November 2020)]. Available online: https://www.who.int/activities/tracking-SARS-CoV-2-variants.
LinkOut - more resources
Full Text Sources
Miscellaneous

