Polyoxa- and Polyazamacrocycles Incorporating 6,7-Diaminoquinoxaline Moiety: Synthesis and Application as Tunable Optical pH-Indicators in Aqueous Solution
- PMID: 36677571
- PMCID: PMC9866286
- DOI: 10.3390/molecules28020512
Polyoxa- and Polyazamacrocycles Incorporating 6,7-Diaminoquinoxaline Moiety: Synthesis and Application as Tunable Optical pH-Indicators in Aqueous Solution
Abstract
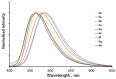
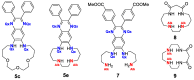
Synthetic approach to fluorescent polyaza- and polyoxadiazamacrocycles comprising a structural fragment of 6,7-diamino-2,3-diphenylquinoxaline has been elaborated using Pd-catalyzed amination providing target compounds in yields up to 77%. A series of nine novel N- and N,O-containing macrocyclic ligands differing by the number of donor sites and cavity size has been obtained. These compounds possess well-pronounced fluorescent properties with emission maxima in a blue region in aprotic solvents and high quantum yields of fluorescence, while in proton media, fluorescence shifts towards the green region of the spectrum. Using macrocycles 5c and 5e as examples, we have shown that such compounds can serve as dual-channel (colorimetric and fluorimetric) pH indicators in water media, with pH transition point and response being dependent on the macrocycle structure due to different sequences of protonation steps.
Keywords: Pd-catalysis; amination; colorimetry; fluorescence; macrocycles; pH indicator; quinoxaline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Brücher E., Baranyai Z., Tircsó G. Biomedical Imaging: The Chemistry of Labels, Probes and Contrast Agents. The Royal Society of Chemistry; Cambridge, UK: 2012. The Future of Biomedical Imaging: Synthesis and Chemical Properties of the DTPA and DOTA Derivative Ligands and Their Complexes; pp. 208–260.
LinkOut - more resources
Full Text Sources

