Xanthomicrol Activity in Cancer HeLa Cells: Comparison with Other Natural Methoxylated Flavones
- PMID: 36677614
- PMCID: PMC9864045
- DOI: 10.3390/molecules28020558
Xanthomicrol Activity in Cancer HeLa Cells: Comparison with Other Natural Methoxylated Flavones
Abstract
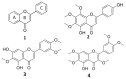
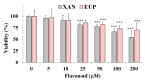
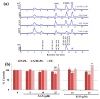
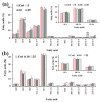
The methoxylated flavone xanthomicrol represents an uncommon active phenolic compound identified in herbs/plants with a long application in traditional medicine. It was isolated from a sample of Achillea erba-rotta subsp. moschata (musk yar-row) flowering tops. Xanthomicrol promising biological properties include antioxidant, anti-inflammatory, antimicrobial, and anticancer activities. This study mainly focused on the evaluation of the xanthomicrol impact on lipid metabolism in cancer HeLa cells, together with the investigation of the treatment-induced changes in cell growth, morphology, and apoptosis. At the dose range of 5-100 μM, xanthomicrol (24 h of incubation) significantly reduced viability and modulated lipid profile in cancer Hela cells. It induced marked changes in the phospholipid/cholesterol ratio, significant decreases in the levels of oleic and palmitic acids, and a marked increase of stearic acid, involving an inhibitory effect on de novo lipogenesis and desaturation in cancer cells. Moreover, marked cell morphological alterations, signs of apoptosis, and cell cycle arrest at the G2/M phase were observed in cancer treated cells. The bioactivity profile of xanthomicrol was compared to that of the anticancer methoxylated flavones eupatilin and artemetin, and structure-activity relationships were underlined.
Keywords: cancer cells; cell cycle; cytotoxicity; lipids; natural flavones; xanthomicrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

