Alkyl 4-Aryl-6-amino-7- phenyl-3-(phenylimino)-4,7-dihydro- 3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxylates: Synthesis and Agrochemical Studies
- PMID: 36677666
- PMCID: PMC9864349
- DOI: 10.3390/molecules28020609
Alkyl 4-Aryl-6-amino-7- phenyl-3-(phenylimino)-4,7-dihydro- 3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxylates: Synthesis and Agrochemical Studies
Abstract
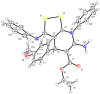
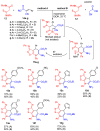
The reaction between dithiomalondianilide (N,N'-diphenyldithiomalondiamide) and alkyl 3-aryl-2-cyanoacrylates in the presence of morpholine in the air atmosphere leads to the formation of alkyl 6-amino-4-aryl-7-phenyl-3-(phenylimino)-4,7-dihydro-3H-[1,2]dithiolo[3,4-b]- pyridine-5-carboxylates in 37-72% yields. The same compounds were prepared in 23-65% yields by ternary condensation of aromatic aldehydes, ethyl(methyl) cyanoacetate and dithiomalondianilide. The reaction mechanism is discussed. The structure of ethyl 6-amino-4-(4-methoxyphenyl)-7-phenyl-3-(phenylimino)-4,7-dihydro-3H-[1,2]dithiolo[3,4-b]pyridine-5-carboxylate was confirmed by X-ray crystallography. Two of the prepared compounds showed a moderate growth-stimulating effect on sunflower seedlings. Three of the new compounds were recognized as strong herbicide safeners with respect to herbicide 2,4-D in the laboratory and field experiments on sunflower.
Keywords: Michael addition; [1,2]dithiolo[3,4-b]pyridines; active methylene thioamides; cyanoacetic esters; dithiomalondianilide; herbicide safeners.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Schmidt U., Kubitzek H. Synthesen mit den Thioamiden der Malonsäure, II. Thiopyridone aus Cyan-thioacetamid. Chem. Ber. 1960;93:1559–1565. doi: 10.1002/cber.19600930716. - DOI
-
- Baggaley K.H. Isothiazolo-Pyridines. GB1560726A. [(accessed on 12 November 2022)];Patent. 1980 February 6; Available online: https://worldwide.espacenet.com/patent/search/family/010090431/publicati....
-
- Baggaley K.H., Jennings L.J.A., Tyrrell A.W.R. Synthesis of 2-substituted isothiazolopyridin-3-ones. J. Heterocycl. Chem. 1982;19:1393–1396. doi: 10.1002/jhet.5570190628. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

