Theoretical Study of the Reaction Mechanism of Phenol-Epoxy Ring-Opening Reaction Using a Latent Hardening Accelerator and a Reactivity Evaluation by Substituents
- PMID: 36677752
- PMCID: PMC9861531
- DOI: 10.3390/molecules28020694
Theoretical Study of the Reaction Mechanism of Phenol-Epoxy Ring-Opening Reaction Using a Latent Hardening Accelerator and a Reactivity Evaluation by Substituents
Abstract
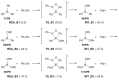
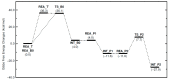
The mechanism of the phenol-epoxide ring-opening reaction using tetraphenylphosphonium-tetraphenylborate (TPP-K) was investigated using the density functional theory (DFT) method. The reaction was initiated by breaking the P-B bond of TPP-K. The generated tetraphenylborate (TetraPB-) reacted with phenol to form a phenoxide ion, which combined with tetraphenylphosphonium (TPP+) to produce the active species, i.e., tetraphenylphosphonium phenolate (TPP-OPh). The phenoxide ion in TPP-OPh nucleophilically attacked the epoxide. Simultaneously, the H atom in the phenolic OH group moved to the O atom of the ring-opened epoxide. The formed phenoxide ion bound to TPP+ again, and TPP-OPh was regenerated. The rate-determining steps in the reaction were the cleavage of the P-B bond and the triphenylborane-forming reaction. The free energies of activation were calculated to be 36.3 and 36.1 kcal/mol, respectively. It is also suggested that these values in the rate-determining steps could be manipulated by substituents introduced on the Ph group of TetraPB-. Based on these results, it is possible to construct new design guidelines for latent hardening accelerators such as TPP-K.
Keywords: density functional theory; latent hardening accelerator; phenol–epoxy ring-opening reaction; tetraphenylphosphonium-tetraphenylborate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


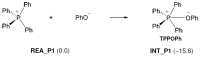

References
-
- Onizuka K. Epoxy Resin Hardener. J. Adhes. Soc. Jpn. 2017;53:122–128. doi: 10.11618/adhesion.53.122. - DOI
-
- Zhao L., Shi Z., Cui Z. Synthesis and characterization of the thermoplastic epoxy phenolic resin for second-order nonlinear optical materials. Mater. Lett. 2012;78:78–196. doi: 10.1016/j.matlet.2012.03.017. - DOI
-
- Kim W.G., Yoon H.G., Lee J.Y. Cure kinetics of biphenyl epoxy resin system using latent catalysts. J. Appl. Polym. Sci. 2001;81:2711–2720. doi: 10.1002/app.1717. - DOI
-
- Zhang X., Zhang L., Zhang D., Liu S., Wei D., Liu F. Mechanism of the temperature-responsive material regulating porous morphology on epoxy phenolic novolac resin microcapsule surface. Colloids Surf. A Physicochem. Eng. Asp. 2020;593:124581. doi: 10.1016/j.colsurfa.2020.124581. - DOI
LinkOut - more resources
Full Text Sources

