Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media
- PMID: 36677877
- PMCID: PMC9864044
- DOI: 10.3390/molecules28020815
Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media
Abstract
Environmental pollution has been a reality for many decades, with its contamination intensifying daily due to rapid urbanization and the ever-increasing world population. Dyes, and especially synthetic ones, constitute a category of pollutants that not only affect the quality of water but also exhibit high toxicity toward living organisms. This study was thoroughly planned to explore the removal of two toxic dyes, namely the methylene blue (MB) and methyl orange (MO) compounds from contaminated aqueous media. For this purpose, we designed and synthesized two new composite materials based on ammonium-functionalized Zr4+ MOF (MOR-1 or UiO-66-NH3+) and naturally occurring sorbents, such as bentonite and clinoptilolite. The composite materials displayed exceptional sorption capability toward both MB+ and MO- ions. A key finding of this study was the high efficiency of the composite materials to simultaneously remove MB+ and MO- under continuous flow conditions, also showing regeneration capability and reusability, thus providing an alternative to well-known mixed bed resins.
Keywords: alginate beads; clay; composite materials; dyes’ sorption; metal–organic frameworks; methyl orange; methylene blue; sorption column; zeolite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



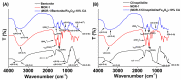

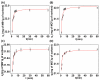





References
-
- Bhatia D., Sharma N.R., Singh J., Kanwar R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017;47:1836–1876. doi: 10.1080/10643389.2017.1393263. - DOI
-
- Rauf M.A., Salman Ashraf S. Survey of recent trends in biochemically assisted degradation of dyes. Chem. Eng. J. 2012;209:520–530. doi: 10.1016/j.cej.2012.08.015. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

