Phytoestrogens and Health Effects
- PMID: 36678189
- PMCID: PMC9864699
- DOI: 10.3390/nu15020317
Phytoestrogens and Health Effects
Abstract
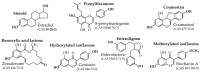
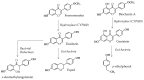
Phytoestrogens are literally estrogenic substances of plant origin. Although these substances are useful for plants in many aspects, their estrogenic properties are essentially relevant to their predators. As such, phytoestrogens can be considered to be substances potentially dedicated to plant-predator interaction. Therefore, it is not surprising to note that the word phytoestrogen comes from the early discovery of estrogenic effects in grazing animals and humans. Here, several compounds whose activities have been discovered at nutritional concentrations in animals and humans are examined. The substances analyzed belong to several chemical families, i.e., the flavanones, the coumestans, the resorcylic acid lactones, the isoflavones, and the enterolignans. Following their definition and the evocation of their role in plants, their metabolic transformations and bioavailabilities are discussed. A point is then made regarding their health effects, which can either be beneficial or adverse depending on the subject studied, the sex, the age, and the physiological status. Toxicological information is given based on official data. The effects are first presented in humans. Animal models are evoked when no data are available in humans. The effects are presented with a constant reference to doses and plausible exposure.
Keywords: bone health; cancer; cardiovascular; health; hypothyroidism; menopause; metabolism; nutrition; phytoestrogens; reproduction; toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Perkin A.G., Newbury F.G. LXXIX.—The colouring matters contained in dyer’s broom (Genista tinctoria) and heather (Calluna vulgaris) J. Chem. Soc. Trans. 1899;75:830–838. doi: 10.1039/CT8997500830. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

