Assessing the Hepatic Safety of Epigallocatechin Gallate (EGCG) in Reproductive-Aged Women
- PMID: 36678191
- PMCID: PMC9861948
- DOI: 10.3390/nu15020320
Assessing the Hepatic Safety of Epigallocatechin Gallate (EGCG) in Reproductive-Aged Women
Abstract
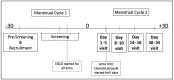
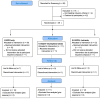
A similar abstract of the interim analysis was previously published in Fertility and Sterility. EPIGALLOCATECHIN GALLATE (EGCG) FOR TREATMENT OF UNEXPLAINED INFERTILITY ASSOCIATED WITH UTERINE FIBROIDS (PRE-FRIEND TRIAL): EARLY SAFETY ASSESSMENT. Uterine fibroids are the most common cause of unexplained infertility in reproductive-aged women. Epigallocatechin gallate (EGCG), a green tea catechin, has demonstrated its ability to shrink uterine fibroids in prior preclinical and clinical studies. Hence, we developed an NICHD Confirm-funded trial to evaluate the use of EGCG for treating women with fibroids and unexplained infertility (FRIEND trial). Prior to embarking on that trial, we here conducted the pre-FRIEND study (NCT04177693) to evaluate the safety of EGCG in premenopausal women. Specifically, our aim was to assess any adverse effects of EGCG alone or in combination with an ovarian stimulator on serum liver function tests (LFTs) and folate level. In this randomized, open-label prospective cohort, participants were recruited from the FRIEND-collaborative clinical sites: Johns Hopkins University, University of Chicago, University of Illinois at Chicago, and Yale University. Thirty-nine women, ages ≥18 to ≤40 years, with/without uterine fibroids, were enrolled and randomized to one of three treatment arms: 800 mg of EGCG daily alone, 800 mg of EGCG daily with clomiphene citrate 100 mg for 5 days, or 800 mg of EGCG daily with Letrozole 5 mg for 5 days. No subject demonstrated signs of drug induced liver injury and no subject showed serum folate level outside the normal range. Hence, our data suggests that a daily dose of 800 mg of EGCG alone or in combination with clomiphene citrate or letrozole (for 5 days) is well-tolerated and is not associated with liver toxicity or folate deficiency in reproductive-aged women.
Keywords: EGCG; dietary factors; epigallocatechin gallate; fibroids; green tea; gynecologic diseases; infertility; leiomyomas; nutrition; safety study; women health.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evaluating the Effect of Epigallocatechin Gallate (EGCG) in Reducing Folate Levels in Reproductive Aged Women by MTHFR and DHFR Genotype in Combination With Letrozole or Clomiphene.Clin Transl Sci. 2025 Mar;18(3):e70189. doi: 10.1111/cts.70189. Clin Transl Sci. 2025. PMID: 40077973 Free PMC article. Clinical Trial.
-
Fibroids and unexplained infertility treatment with epigallocatechin gallate: a natural compound in green tea (FRIEND) - protocol for a randomised placebo-controlled US multicentre clinical trial of EGCG to improve fertility in women with uterine fibroids.BMJ Open. 2024 Jan 12;14(1):e078989. doi: 10.1136/bmjopen-2023-078989. BMJ Open. 2024. PMID: 38216200 Free PMC article.
-
Biological and Mechanistic Characterization of Novel Prodrugs of Green Tea Polyphenol Epigallocatechin Gallate Analogs in Human Leiomyoma Cell Lines.J Cell Biochem. 2016 Oct;117(10):2357-69. doi: 10.1002/jcb.25533. Epub 2016 Mar 28. J Cell Biochem. 2016. PMID: 26950525
-
Epigallocatechin Gallate for the Treatment of Benign and Malignant Gynecological Diseases-Focus on Epigenetic Mechanisms.Nutrients. 2024 Feb 17;16(4):559. doi: 10.3390/nu16040559. Nutrients. 2024. PMID: 38398883 Free PMC article. Review.
-
Green Tea and Benign Gynecologic Disorders: A New Trick for An Old Beverage?Nutrients. 2023 Mar 16;15(6):1439. doi: 10.3390/nu15061439. Nutrients. 2023. PMID: 36986169 Free PMC article. Review.
Cited by
-
The Role of Green Tea Catechin Epigallocatechin Gallate (EGCG) and Mammalian Target of Rapamycin (mTOR) Inhibitor PP242 (Torkinib) in the Treatment of Spinal Cord Injury.Antioxidants (Basel). 2023 Feb 3;12(2):363. doi: 10.3390/antiox12020363. Antioxidants (Basel). 2023. PMID: 36829922 Free PMC article.
-
The Effects of Natural Epigenetic Therapies in 3D Ovarian Cancer and Patient-Derived Tumor Explants: New Avenues in Regulating the Cancer Secretome.Biomolecules. 2023 Jul 1;13(7):1066. doi: 10.3390/biom13071066. Biomolecules. 2023. PMID: 37509102 Free PMC article.
-
The potential of epigallocatechin gallate in the chemoprevention and therapy of hepatocellular carcinoma.Front Pharmacol. 2023 May 24;14:1201085. doi: 10.3389/fphar.2023.1201085. eCollection 2023. Front Pharmacol. 2023. PMID: 37292151 Free PMC article. Review.
-
Calorie restriction mimetics against aging and inflammation.Biogerontology. 2025 Jun 24;26(4):126. doi: 10.1007/s10522-025-10269-0. Biogerontology. 2025. PMID: 40553215 Review.
-
Epigallocatechin gallate attenuated high glucose-induced pancreatic beta cell dysfunction by modulating DRP1-mediated mitochondrial apoptosis pathways.Sci Rep. 2024 Jul 22;14(1):16809. doi: 10.1038/s41598-024-67867-0. Sci Rep. 2024. PMID: 39039202 Free PMC article.
References
-
- ElKafas H., Ali M., Al-Hendy A. Encyclopedia of Reproduction. Academic Press; Cambridge, MA, USA: 2018. Leiomyomas; pp. 101–105.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

