Novel Therapeutic Potentials of Taxifolin for Obesity-Induced Hepatic Steatosis, Fibrogenesis, and Tumorigenesis
- PMID: 36678220
- PMCID: PMC9865844
- DOI: 10.3390/nu15020350
Novel Therapeutic Potentials of Taxifolin for Obesity-Induced Hepatic Steatosis, Fibrogenesis, and Tumorigenesis
Abstract
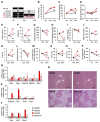
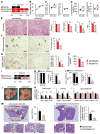
The molecular pathogenesis of nonalcoholic steatohepatitis (NASH) includes a complex interaction of metabolic stress and inflammatory stimuli. Considering the therapeutic goals of NASH, it is important to determine whether the treatment can prevent the progression from NASH to hepatocellular carcinoma. Taxifolin, also known as dihydroquercetin, is a natural bioactive flavonoid with antioxidant and anti-inflammatory properties commonly found in various foods and health supplement products. In this study, we demonstrated that Taxifolin treatment markedly prevented the development of hepatic steatosis, chronic inflammation, and liver fibrosis in a murine model of NASH. Its mechanisms include a direct action on hepatocytes to inhibit lipid accumulation. Taxifolin also increased brown adipose tissue activity and suppressed body weight gain through at least two distinct pathways: direct action on brown adipocytes and indirect action via fibroblast growth factor 21 production in the liver. Notably, the Taxifolin treatment after NASH development could effectively prevent the development of liver tumors. Collectively, this study provides evidence that Taxifolin shows pleiotropic effects for the treatment of the NASH continuum. Our data also provide insight into the novel mechanisms of action of Taxifolin, which has been widely used as a health supplement with high safety.
Keywords: Taxifolin; antioxidant; brown adipocytes; fibroblast growth factor-21; inflammation; nonalcoholic steatohepatitis (NASH); obesity.
Conflict of interest statement
The authors declare no conflict of interest. The funding agencies had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Seaknit Biotechnology Co., Ltd., donated; Department of Metabolic Syndrome and Nutritional Science, Research Institute of Environmental Medicine, Nagoya University.
Figures






References
-
- Yoshioka N., Tanaka M., Ochi K., Watanabe A., Ono K., Sawada M., Ogi T., Itoh M., Ito A., Shiraki Y., et al. The sodium-glucose cotransporter-2 inhibitor Tofogliflozin prevents the progression of nonalcoholic steatohepatitis-associated liver tumors in a novel murine model. Biomed. Pharmacother. 2021;140:111738. doi: 10.1016/j.biopha.2021.111738. - DOI - PubMed
-
- Kawakubo M., Tanaka M., Ochi K., Watanabe A., Saka-Tanaka M., Kanamori Y., Yoshioka N., Yamashita S., Goto M., Itoh M., et al. Dipeptidyl peptidase-4 inhibition prevents nonalcoholic steatohepatitis-associated liver fibrosis and tumor development in mice independently of its anti-diabetic effects. Sci. Rep. 2020;10:983. doi: 10.1038/s41598-020-57935-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 18H02737/Japan Society for the Promotion of Science
- 18K19769/Japan Society for the Promotion of Science
- 19K07905/Japan Society for the Promotion of Science
- 20H03447/Japan Society for the Promotion of Science
- 21H02835/Japan Society for the Promotion of Science
- 21K08526/Japan Society for the Promotion of Science
- 22H04806/Japan Society for the Promotion of Science
- 22K11720/Japan Society for the Promotion of Science
- 22K19524/Japan Society for the Promotion of Science
- 22K19723/Japan Society for the Promotion of Science
- JP22gm1210009s0104/Japan Agency for Medical Research and Development
- JP22fk0210082s0102/Japan Agency for Medical Research and Development
- 22fk0210094s0202/Japan Agency for Medical Research and Development
- JP22ek0109488/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources
Medical

