Sea Cucumber Derived Triterpenoid Glycoside Frondoside A: A Potential Anti-Bladder Cancer Drug
- PMID: 36678249
- PMCID: PMC9861588
- DOI: 10.3390/nu15020378
Sea Cucumber Derived Triterpenoid Glycoside Frondoside A: A Potential Anti-Bladder Cancer Drug
Abstract
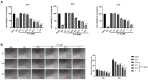
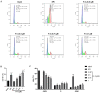
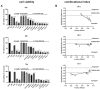
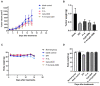
Bladder cancer is a highly recurrent disease and a common cause of cancer-related deaths worldwide. Despite recent developments in diagnosis and therapy, the clinical outcome of bladder cancer remains poor; therefore, novel anti-bladder cancer drugs are urgently needed. Natural bioactive substances extracted from marine organisms such as sea cucumbers, scallops, and sea urchins are believed to have anti-cancer activity with high effectiveness and less toxicity. Frondoside A is a triterpenoid glycoside isolated from sea cucumber, Cucumaria frondosa. It has been demonstrated that Frondoside A exhibits anti-proliferative, anti-invasive, anti-angiogenic, anti-cancer, and potent immunomodulatory effects. In addition, CpG oligodeoxynucleotide (CpG-ODN) has also been shown to have potent anti-cancer effects in various tumors models, such as liver cancer, breast cancer, and bladder cancer. However, very few studies have investigated the effectiveness of Frondoside A against bladder cancer alone or in combination with CpG-ODN. In this study, we first investigated the individual effects of both Frondoside A and CpG-ODN and subsequently studied their combined effects on human bladder cancer cell viability, migration, apoptosis, and cell cycle in vitro, and on tumor growth in nude mice using human bladder cancer cell line UM-UC-3. To interrogate possible synergistic effects, combinations of different concentrations of the two drugs were used. Our data showed that Frondoside A decreased the viability of bladder cancer cells UM-UC-3 in a concentration-dependent manner, and its inhibitory effect on cell viability (2.5 μM) was superior to EPI (10 μM). We also showed that Frondoside A inhibited UM-UC-3 cell migration, affected the distribution of cell cycle and induced cell apoptosis in concentration-dependent manners, which effectively increased the sub-G1 (apoptotic) cell fraction. In addition, we also demonstrated that immunomodulator CpG-ODN could synergistically potentiate the inhibitory effects of Frondoside A on the proliferation and migration of human bladder cancer cell line UM-UC-3. In in vivo experiments, Frondoside A (800 μg/kg/day i.p. for 14 days) alone and in combination with CpG-ODN (1 mg/kg/dose i.p.) significantly decreased the growth of UM-UC-3 tumor xenografts, without any significant toxic side-effects; however, the chemotherapeutic agent EPI caused weight loss in nude mice. Taken together, these findings indicated that Frondoside A in combination with CpG-ODN is a promising therapeutic strategy for bladder cancer.
Keywords: CpG oligodexynucleotides; Frondoside A; apoptosis; bladder cancer; cell migration; cell viability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts.Eur J Pharmacol. 2011 Oct 1;668(1-2):25-34. doi: 10.1016/j.ejphar.2011.06.023. Epub 2011 Jun 27. Eur J Pharmacol. 2011. PMID: 21741966
-
Frondoside A is a potential anticancer agent from sea cucumbers.J Cancer Res Ther. 2019 Jul-Sep;15(5):953-960. doi: 10.4103/jcrt.JCRT_1427_16. J Cancer Res Ther. 2019. PMID: 31603094 Review.
-
Frondoside a suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis.PLoS One. 2013;8(1):e53087. doi: 10.1371/journal.pone.0053087. Epub 2013 Jan 8. PLoS One. 2013. Retraction in: PLoS One. 2023 Sep 8;18(9):e0291478. doi: 10.1371/journal.pone.0291478. PMID: 23308143 Free PMC article. Retracted.
-
The marine triterpene glycoside frondoside A induces p53-independent apoptosis and inhibits autophagy in urothelial carcinoma cells.BMC Cancer. 2017 Feb 1;17(1):93. doi: 10.1186/s12885-017-3085-z. BMC Cancer. 2017. PMID: 28143426 Free PMC article.
-
The Anti-Cancer Effects of Frondoside A.Mar Drugs. 2018 Feb 19;16(2):64. doi: 10.3390/md16020064. Mar Drugs. 2018. PMID: 29463049 Free PMC article. Review.
Cited by
-
Saponins of North Atlantic Sea Cucumber: Chemistry, Health Benefits, and Future Prospectives.Mar Drugs. 2023 Apr 23;21(5):262. doi: 10.3390/md21050262. Mar Drugs. 2023. PMID: 37233456 Free PMC article. Review.
-
Mechanisms of Action of Sea Cucumber Triterpene Glycosides Cucumarioside A0-1 and Djakonovioside A Against Human Triple-Negative Breast Cancer.Mar Drugs. 2024 Oct 17;22(10):474. doi: 10.3390/md22100474. Mar Drugs. 2024. PMID: 39452882 Free PMC article.
-
TRAIL-mediated signaling in bladder cancer: realization of clinical efficacy of TRAIL-based therapeutics in medical oncology.Med Oncol. 2023 Jul 11;40(8):236. doi: 10.1007/s12032-023-02078-7. Med Oncol. 2023. PMID: 37432489 Review.
-
Advancements and challenges in pharmacokinetic and pharmacodynamic research on the traditional Chinese medicine saponins: a comprehensive review.Front Pharmacol. 2024 May 7;15:1393409. doi: 10.3389/fphar.2024.1393409. eCollection 2024. Front Pharmacol. 2024. PMID: 38774213 Free PMC article. Review.
-
Marine-Derived Compounds: A New Horizon in Cancer, Renal, and Metabolic Disease Therapeutics.Mar Drugs. 2025 Jul 9;23(7):283. doi: 10.3390/md23070283. Mar Drugs. 2025. PMID: 40710508 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

