Sodium Homeostasis, a Balance Necessary for Life
- PMID: 36678265
- PMCID: PMC9862583
- DOI: 10.3390/nu15020395
Sodium Homeostasis, a Balance Necessary for Life
Abstract
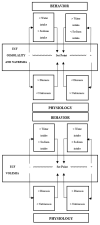
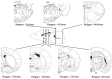
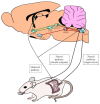
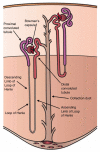
Body sodium (Na) levels must be maintained within a narrow range for the correct functioning of the organism (Na homeostasis). Na disorders include not only elevated levels of this solute (hypernatremia), as in diabetes insipidus, but also reduced levels (hyponatremia), as in cerebral salt wasting syndrome. The balance in body Na levels therefore requires a delicate equilibrium to be maintained between the ingestion and excretion of Na. Salt (NaCl) intake is processed by receptors in the tongue and digestive system, which transmit the information to the nucleus of the solitary tract via a neural pathway (chorda tympani/vagus nerves) and to circumventricular organs, including the subfornical organ and area postrema, via a humoral pathway (blood/cerebrospinal fluid). Circuits are formed that stimulate or inhibit homeostatic Na intake involving participation of the parabrachial nucleus, pre-locus coeruleus, medial tuberomammillary nuclei, median eminence, paraventricular and supraoptic nuclei, and other structures with reward properties such as the bed nucleus of the stria terminalis, central amygdala, and ventral tegmental area. Finally, the kidney uses neural signals (e.g., renal sympathetic nerves) and vascular (e.g., renal perfusion pressure) and humoral (e.g., renin-angiotensin-aldosterone system, cardiac natriuretic peptides, antidiuretic hormone, and oxytocin) factors to promote Na excretion or retention and thereby maintain extracellular fluid volume. All these intake and excretion processes are modulated by chemical messengers, many of which (e.g., aldosterone, angiotensin II, and oxytocin) have effects that are coordinated at peripheral and central level to ensure Na homeostasis.
Keywords: excitatory and inhibitory circuits; hypernatremia; hyponatremia; kidney; natriuresis; posterior hypothalamus; salt intake; sodium homeostasis; taste.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Neurochemical Circuits Subserving Fluid Balance and Baroreflex: A Role for Serotonin, Oxytocin, and Gonadal Steroids.In: De Luca LA Jr, Menani JV, Johnson AK, editors. Neurobiology of Body Fluid Homeostasis: Transduction and Integration. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 9. In: De Luca LA Jr, Menani JV, Johnson AK, editors. Neurobiology of Body Fluid Homeostasis: Transduction and Integration. Boca Raton (FL): CRC Press/Taylor & Francis; 2014. Chapter 9. PMID: 24829993 Free Books & Documents. Review.
-
Activation of renal afferent pathways following furosemide treatment. II. Effect Of angiotensin blockade.Brain Res. 2000 Apr 10;861(2):377-89. doi: 10.1016/s0006-8993(00)02049-7. Brain Res. 2000. PMID: 10760499
-
Brain regions influenced by the lateral parabrachial nucleus in angiotensin II-induced water intake.Neuroscience. 2013 Nov 12;252:410-9. doi: 10.1016/j.neuroscience.2013.08.027. Epub 2013 Aug 27. Neuroscience. 2013. PMID: 23994596
-
Angiotensin, thirst, and sodium appetite.Physiol Rev. 1998 Jul;78(3):583-686. doi: 10.1152/physrev.1998.78.3.583. Physiol Rev. 1998. PMID: 9674690 Review.
-
Brain angiotensin and body fluid homeostasis.Jpn J Physiol. 2001 Jun;51(3):281-9. doi: 10.2170/jjphysiol.51.281. Jpn J Physiol. 2001. PMID: 11492952 Review.
Cited by
-
Sodium Retention in Large Herbivores: Physiological Insights and Zoogeochemical Consequences.J Exp Zool A Ecol Integr Physiol. 2025 Jul;343(6):664-676. doi: 10.1002/jez.2924. Epub 2025 Apr 17. J Exp Zool A Ecol Integr Physiol. 2025. PMID: 40247661 Free PMC article.
-
Dietary salt in liver cirrhosis: With a pinch of salt!World J Hepatol. 2023 Oct 27;15(10):1084-1090. doi: 10.4254/wjh.v15.i10.1084. World J Hepatol. 2023. PMID: 37970619 Free PMC article. Review.
-
Effect of low sodium and high potassium diet on lowering blood pressure and cardiovascular events.Clin Hypertens. 2024 Jan 2;30(1):2. doi: 10.1186/s40885-023-00259-0. Clin Hypertens. 2024. PMID: 38163867 Free PMC article. Review.
-
Modelling hemodynamics regulation in rats and dogs to facilitate drugs safety risk assessment.Front Pharmacol. 2024 Oct 29;15:1402462. doi: 10.3389/fphar.2024.1402462. eCollection 2024. Front Pharmacol. 2024. PMID: 39534082 Free PMC article.
-
Effects of reduced extracellular sodium on proliferation and invasive activity of renal cell carcinoma cell lines.Sci Rep. 2025 Mar 8;15(1):8067. doi: 10.1038/s41598-025-92674-6. Sci Rep. 2025. PMID: 40055463 Free PMC article.
References
-
- Schulkin J. Sodium Hunger: The Search for a Salty Taste. Cambridge University Press; Cambridge, UK: 1991.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

