Antimicrobial Resistance and Genetic Diversity of Pseudomonas aeruginosa Strains Isolated from Equine and Other Veterinary Samples
- PMID: 36678412
- PMCID: PMC9867525
- DOI: 10.3390/pathogens12010064
Antimicrobial Resistance and Genetic Diversity of Pseudomonas aeruginosa Strains Isolated from Equine and Other Veterinary Samples
Abstract
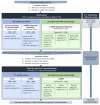
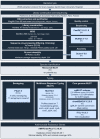
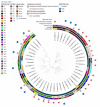
Pseudomonas aeruginosa is one of the leading causes of healthcare-associated infections in humans. This bacterium is less represented in veterinary medicine, despite causing difficult-to-treat infections due to its capacity to acquire antimicrobial resistance, produce biofilms, and persist in the environment, along with its limited number of veterinary antibiotic therapies. Here, we explored susceptibility profiles to antibiotics and to didecyldimethylammonium chloride (DDAC), a quaternary ammonium widely used as a disinfectant, in 168 P. aeruginosa strains isolated from animals, mainly Equidae. A genomic study was performed on 41 of these strains to determine their serotype, sequence type (ST), relatedness, and resistome. Overall, 7.7% of animal strains were resistant to carbapenems, 10.1% presented a multidrug-resistant (MDR) profile, and 11.3% showed decreased susceptibility (DS) to DDAC. Genomic analyses revealed that the study population was diverse, and 4.9% were ST235, which is considered the most relevant human high-risk clone worldwide. This study found P. aeruginosa populations with carbapenem resistance, multidrug resistance, and DS to DDAC in equine and canine isolates. These strains, which are not susceptible to antibiotics used in veterinary and human medicine, warrant close the setting up of a clone monitoring, based on that already in place in human medicine, in a one-health approach.
Keywords: Pseudomonas aeruginosa; antimicrobial susceptibility; equine; resistome; whole-genome sequencing and typing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control . Antimicrobial Resistance Surveillance in Europe: 2022–2020 Data. WHO Regional Office for Europe; Copenhagen, Denmark: 2022. p. 164.
-
- Résapath . Réseau d’Epidémiosurveillance de l’Antibiorésistance des Bactéries Pathogènes Animales, Bilan 2014. Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (Anses); Lyon, France: Ploufragan-Plouzané-Niort Laboratory; Ploufragan, France: 2015. p. 168.
Grants and funding
LinkOut - more resources
Full Text Sources

