Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma
- PMID: 36678534
- PMCID: PMC9864417
- DOI: 10.3390/ph16010037
Afatinib Reverses EMT via Inhibiting CD44-Stat3 Axis to Promote Radiosensitivity in Nasopharyngeal Carcinoma
Abstract
Background: Afatinib, a second-generation tyrosine kinase inhibitor (TKI), exerts its radiosensitive effects in nasopharyngeal carcinoma (NPC). However, the detailed mechanism of afatinib-mediated sensitivity to radiation is still obscure in NPC.
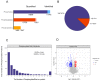
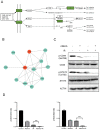
Methods: Quantitative phosphorylated proteomics and bioinformatics analysis were performed to illustrate the global phosphoprotein changes. The activity of the CD44-Stat3 axis and Epithelial-Mesenchymal Transition (EMT)-linked markers were evaluated by Western blotting. Wound healing and transwell assays were used to determine the levels of cell migration upon afatinib combined IR treatment. Cell proliferation was tested by CCK-8 assay. A pharmacological agonist by IL-6 was applied to activate Stat3. The xenograft mouse model was treated with afatinib, radiation or a combination of afatinib and radiation to detect the radiosensitivity of afatinib in vivo.
Results: In the present study, we discovered that afatinib triggered global protein phosphorylation alterations in NPC cells. Further, bioinformatics analysis indicated that afatinib inhibited the CD44-Stat3 signaling and subsequent EMT process. Moreover, functional assays demonstrated that afatinib combined radiation treatment remarkably impeded cell viability, migration, EMT process and CD44-Stat3 activity in vitro and in vivo. In addition, pharmacological stimulation of Stat3 rescued radiosensitivity and biological functions induced by afatinib in NPC cells. This suggested that afatinib reversed the EMT process by blocking the activity of the CD44-Stat3 axis.
Conclusion: Collectively, this work identifies the molecular mechanism of afatinib as a radiation sensitizer, thus providing a potentially useful combination treatment and drug target for NPC radiosensitization. Our findings describe a new function of afatinib in radiosensitivity and cancer treatment.
Keywords: CD44-Stat3 signaling pathway; afatinib; epithelial-to-mesenchymal transition (EMT); nasopharyngeal carcinoma (NPC); radiosensitivity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

