Imaging of the Glucose-Dependent Insulinotropic Polypeptide Receptor Using a Novel Radiolabeled Peptide Rationally Designed Based on Endogenous GIP and Synthetic Exendin-4 Sequences
- PMID: 36678558
- PMCID: PMC9864903
- DOI: 10.3390/ph16010061
Imaging of the Glucose-Dependent Insulinotropic Polypeptide Receptor Using a Novel Radiolabeled Peptide Rationally Designed Based on Endogenous GIP and Synthetic Exendin-4 Sequences
Abstract
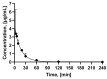
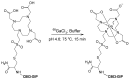
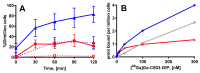
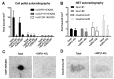
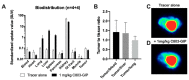
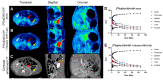
Imaging and radiotherapy targeting the glucose-dependent insulinotropic polypeptide receptor (GIPR) could potentially benefit the management of neuroendocrine neoplasms (NENs), complementing clinically established radiopharmaceuticals. The aim of this study was to evaluate a GIPR-targeting positron emission tomography (PET) radioligand with receptor-specific binding, fast blood clearance, and low liver background uptake. The peptide DOTA-bioconjugate, C803-GIP, was developed based on the sequence of the endogenous GIP(1-30) and synthetic exendin-4 peptides with selective amino acid mutations to combine their specificity for the GIPR and in vivo stability, respectively. The 68Ga-labeled bioconjugate was evaluated in vitro in terms of binding affinity, specificity, and internalization in HEK293 cells transfected with the human GIPR, GLP1, or GCG receptors and in sections of human insulinoma and NENs. In vivo binding specificity, biodistribution, and tissue background were investigated in mice bearing huGIPR-HEK293 xenografts and in a pig. Ex vivo organ distribution, pharmacokinetics, and dosimetry were studied in normal rats. [68Ga]Ga-C803-GIP was stable and demonstrated a high affinity to the huGIPR-HEK293 cells. Binding specificity was demonstrated in vitro in frozen sections of NENs and huGIPR-HEK293 cells. No specific uptake was observed in the negative controls of huGLP1R and huGCGR cells. A novel rationally designed PET radioligand, [68Ga]Ga-C803-GIP, demonstrated promising binding characteristics and specificity towards the GIPR.
Keywords: GIPR; PET; insulinoma; neuroendocrine tumors.
Conflict of interest statement
Torsten Haack, Martin Bossart, and Michael Wagner are employees of Sanofi-Aventis and may hold shares and/or stock options in the company. Olof Eriksson, Iina Laitinen, Stefan Pierrou, and Lars Johansson are employees of Antaros Medical AB. No other potential conflict of interest relevant to this article exist.
Figures

Similar articles
-
Introduction of a fatty acid chain modification to prolong circulatory half-life of a radioligand towards glucose-dependent insulinotropic polypeptide receptor.Nucl Med Biol. 2024 Jan-Feb;128-129:108876. doi: 10.1016/j.nucmedbio.2024.108876. Epub 2024 Jan 10. Nucl Med Biol. 2024. PMID: 38241936
-
Drug Occupancy Assessment at the Glucose-Dependent Insulinotropic Polypeptide Receptor by Positron Emission Tomography.Diabetes. 2021 Apr;70(4):842-853. doi: 10.2337/db20-1096. Epub 2021 Feb 5. Diabetes. 2021. PMID: 33547046
-
The glucose-dependent insulinotropic polypeptide receptor: a novel target for neuroendocrine tumor imaging—first preclinical studies.J Nucl Med. 2014 Jun;55(6):976-82. doi: 10.2967/jnumed.113.133744. Epub 2014 Apr 17. J Nucl Med. 2014. PMID: 24744444
-
Molecular evolution of GIP and Exendin and their receptors.Peptides. 2020 Mar;125:170158. doi: 10.1016/j.peptides.2019.170158. Epub 2019 Sep 30. Peptides. 2020. PMID: 31582191 Review.
-
Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents.Peptides. 2018 Feb;100:173-181. doi: 10.1016/j.peptides.2017.11.021. Peptides. 2018. PMID: 29412817 Review.
Cited by
-
Glucose-dependent insulinotropic polypeptide (GIP).Mol Metab. 2025 May;95:102118. doi: 10.1016/j.molmet.2025.102118. Epub 2025 Feb 28. Mol Metab. 2025. PMID: 40024571 Free PMC article. Review.
-
Clinical application of targeted α-emitter therapy in gastroenteropancreatic neuroendocrine neoplasms.J Gastroenterol. 2025 Jul;60(7):809-819. doi: 10.1007/s00535-025-02241-z. Epub 2025 Apr 12. J Gastroenterol. 2025. PMID: 40220045 Free PMC article. Review.
-
The historical progression of positron emission tomography research in neuroendocrinology.Front Neuroendocrinol. 2023 Jul;70:101081. doi: 10.1016/j.yfrne.2023.101081. Epub 2023 Jul 7. Front Neuroendocrinol. 2023. PMID: 37423505 Free PMC article.
References
-
- Yaqub T., Tikhonova I.G., Lattig J., Magnan R., Laval M., Escrieut C., Boulegue C., Hewage C., Fourmy D. Identification of determinants of glucose-dependent insulinotropic polypeptide receptor that interact with N-terminal biologically active region of the natural ligand. Mol. Pharmacol. 2010;77:547–558. doi: 10.1124/mol.109.060111. - DOI - PubMed
LinkOut - more resources
Full Text Sources

