Cardiovascular Toxicities of Ibrutinib: A Pharmacovigilance Study Based on the United States Food and Drug Administration Adverse Event Reporting System Database
- PMID: 36678594
- PMCID: PMC9863914
- DOI: 10.3390/ph16010098
Cardiovascular Toxicities of Ibrutinib: A Pharmacovigilance Study Based on the United States Food and Drug Administration Adverse Event Reporting System Database
Abstract
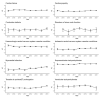
Background: Although ibrutinib has been widely used to treat haematological malignancies, many studies have reported associated cardiovascular events. These studies were primarily animal experiments and clinical trials. For more rational clinical drug use, a study based on post-marketing data is necessary. Aim: Based on post-marketing data, we investigated the clinical features, time to onset, and outcomes of potential cardiovascular toxicities of ibrutinib. Methods: This disproportionality study utilised data from the 2014−2021 United States Food and Drug Administration Adverse Event Reporting System (FAERS) database. We used two disproportionality methods information component (IC) and reporting odds ratio (ROR)) to detect the potential cardiovascular toxicities of ibrutinib. Positive signals were defined as IC025 > 0 and ROR025 > 1. Results: A total of 10 cardiovascular events showed positive signals: supraventricular tachyarrhythmias, haemorrhagic central nervous system vascular conditions, ventricular tachyarrhythmias, cardiac failure, ischaemic central nervous system vascular conditions, cardiomyopathy, conduction defects, myocardial infarction, myocardial infarction disorders of sinus node function, and torsade de pointes/QT prolongation. Cardiomyopathy and supraventricular tachyarrhythmias were the two most common signals. Disorders of sinus node function were observed for the first time, which may be a new adverse effect of ibrutinib. Conclusions: This pharmacovigilance study systematically explored the adverse cardiovascular events of ibrutinib and provided new safety signals based on past safety information. Attention should be paid to some high-risk signals.
Keywords: FAERS; cardiovascular events; disproportionality analysis; ibrutinib; pharmacovigilance study.
Conflict of interest statement
We confirm that the authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures
Similar articles
-
Toxicity signals associated with secukinumab: A pharmacovigilance study based on the United States Food and Drug Administration Adverse Event Reporting System database.Br J Clin Pharmacol. 2023 Feb;89(2):865-873. doi: 10.1111/bcp.15535. Epub 2022 Oct 7. Br J Clin Pharmacol. 2023. PMID: 36106653
-
Cardiovascular Toxicities Associated With Ibrutinib.J Am Coll Cardiol. 2019 Oct 1;74(13):1667-1678. doi: 10.1016/j.jacc.2019.07.056. J Am Coll Cardiol. 2019. PMID: 31558250
-
Data mining and analysis for emicizumab adverse event signals based on the Food and Drug Administration Adverse Event Reporting System database.Int J Clin Pharm. 2023 Jun;45(3):622-629. doi: 10.1007/s11096-022-01514-4. Epub 2023 Feb 27. Int J Clin Pharm. 2023. PMID: 36848023
-
Cardiovascular safety of rapidly accelerated fibrosarcoma B-type and/or mitogen-activated extracellular signal-regulated kinase inhibitors: A mixed approach combining a meta-analysis and a pharmacovigilance disproportionality analysis.Arch Cardiovasc Dis. 2020 Jun-Jul;113(6-7):420-432. doi: 10.1016/j.acvd.2020.03.014. Epub 2020 May 1. Arch Cardiovasc Dis. 2020. PMID: 32418884
-
SGLT2 inhibitors associated pancreatitis: signal identification through disproportionality analysis of spontaneous reports and review of case reports.Int J Clin Pharm. 2022 Dec;44(6):1425-1433. doi: 10.1007/s11096-022-01476-7. Epub 2022 Oct 12. Int J Clin Pharm. 2022. PMID: 36224513 Review.
Cited by
-
Neurological adverse events associated with baclofen: A disproportionality analysis based on FDA Adverse Event Reporting System.SAGE Open Med. 2025 Apr 28;13:20503121251331812. doi: 10.1177/20503121251331812. eCollection 2025. SAGE Open Med. 2025. PMID: 40303631 Free PMC article.
-
Epidemiology, clinical characteristics and potential mechanism of ibrutinib-induced ventricular arrhythmias.Front Pharmacol. 2024 Nov 19;15:1513913. doi: 10.3389/fphar.2024.1513913. eCollection 2024. Front Pharmacol. 2024. PMID: 39629084 Free PMC article. Review.
-
Which is the top player for the cardiovascular safety? ibrutinib vs. obinutuzumab in CLL.Front Pharmacol. 2023 Aug 16;14:1229304. doi: 10.3389/fphar.2023.1229304. eCollection 2023. Front Pharmacol. 2023. PMID: 37654615 Free PMC article.
-
Chronic Lymphocytic Leukemia Care and Beyond: Navigating the Needs of Long-Term Survivors.Cancers (Basel). 2025 Jan 2;17(1):119. doi: 10.3390/cancers17010119. Cancers (Basel). 2025. PMID: 39796746 Free PMC article. Review.
-
Impaired Cardiac AMPK (5'-Adenosine Monophosphate-Activated Protein Kinase) and Ca2+-Handling, and Action Potential Duration Heterogeneity in Ibrutinib-Induced Ventricular Arrhythmia Vulnerability.J Am Heart Assoc. 2024 Jun 18;13(12):e032357. doi: 10.1161/JAHA.123.032357. Epub 2024 Jun 6. J Am Heart Assoc. 2024. PMID: 38842296 Free PMC article.
References
Grants and funding
- No. 82073671/the National Nature Science Foundation of China
- No. GWV-10.2-XD22/the Leading Talents of Public Health in Shanghai
- No. GWV-10.2-YQ33/the Excellent Young Scholars of public health in Shanghai
- GWV-10.1-XK05/three-year Action Program of Shanghai Municipality for Strengthening the Construction of Public Health System
- No.03/Big Data and Artificial Intelligence Application,and Military Key Discipline Construction Project (Health Service-Naval Health Service Organization and Command)
LinkOut - more resources
Full Text Sources
Miscellaneous