Engineered Exosomes for Tumor-Targeted Drug Delivery: A Focus on Genetic and Chemical Functionalization
- PMID: 36678695
- PMCID: PMC9865907
- DOI: 10.3390/pharmaceutics15010066
Engineered Exosomes for Tumor-Targeted Drug Delivery: A Focus on Genetic and Chemical Functionalization
Abstract
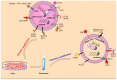
Cancer is the main cause of death worldwide. The limitations in traditional cancer therapies provoked the advance and use of several nanotechnologies for more effective and nontoxic cancer treatment. Along with synthetic nanocarriers, extracellular vesicles (EVs)-mediated drug delivery systems have aroused substantial interest. The term EVs refers to cell-derived nanovesicles, such as exosomes, with phospholipid-bound structures, participating in cell-to-cell communication. Exosomes are 30-150 nm vesicles that can transfer many biological molecules between cells. From a drug delivery standpoint, exosomes can be loaded with various therapeutic cargo, with the several advantages of low immunogenicity, high biocompatibility, transformative, and effective tumor targeting aptitude. The exosomal surface can be functionalized to improve tumor targeting ability of them. Researchers have genetically expressed or chemically linked various molecules on the surface of exosomes. Despite extensive investigation, clinical translation of exosome-based drug delivery remains challenging. In this review, we discuss various methods used to loading exosomes with therapeutic cargo. We describe examples of functionalized exosomes surface using genetic and chemical modification methods. Finally, this review attempts to provide future outlooks for exosome-based targeted drug delivery.
Keywords: drug delivery; exosomes; extracellular vesicles; nanocarriers.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures




References
-
- Cantini L., Mentrasti G., Russo G.L., Signorelli D., Pasello G., Rijavec E., Russano M., Antonuzzo L., Rocco D., Giusti R., et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): Fewer cases and higher stages from a real-world scenario. ESMO Open. 2022;7:100406. doi: 10.1016/j.esmoop.2022.100406. - DOI - PMC - PubMed
-
- Bao Y., Liu S., Zhou Q., Cai P., Anfossi S., Li Q., Hu Y., Liu M., Fu J., Rong T. Three-dimensional conformal radiotherapy with concurrent chemotherapy for postoperative recurrence of esophageal squamous cell carcinoma: Clinical efficacy and failure pattern. Radiat. Oncol. 2013;8:1–8. doi: 10.1186/1748-717X-8-241. - DOI - PMC - PubMed
-
- Yagawa Y., Tanigawa K., Kobayashi Y., Yamamoto M. Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J. Cancer Metastasis Treat. 2017;3:218–230. doi: 10.20517/2394-4722.2017.35. - DOI
Publication types
LinkOut - more resources
Full Text Sources

