A Supramolecular Nanoassembly of Lenvatinib and a Green Light-Activatable NO Releaser for Combined Chemo-Phototherapy
- PMID: 36678725
- PMCID: PMC9865831
- DOI: 10.3390/pharmaceutics15010096
A Supramolecular Nanoassembly of Lenvatinib and a Green Light-Activatable NO Releaser for Combined Chemo-Phototherapy
Abstract
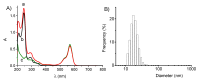
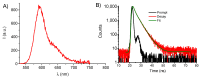
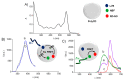
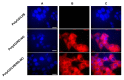
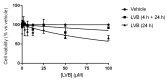
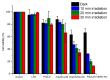
The chemotherapeutic Lenvatinib (LVB) and a nitric oxide (NO) photodonor based on a rhodamine antenna (RD-NO) activatable by the highly compatible green light are supramolecularly assembled by a β-cyclodextrin branched polymer (PolyCD). The poorly water-soluble LVB and RD-NO solubilize very well within the polymeric host leading to a ternary supramolecular nanoassembly with a diameter of ~55 nm. The efficiency of the NO photorelease and the typical red fluorescence of RD-NO significantly enhance within the polymer due to its active role in the photochemical and photophysical deactivation pathways. The co-presence of LVB within the same host does not affect either the nature or the efficiency of the photoinduced processes of RD-NO. Besides, irradiation of RD-NO does not lead to the decomposition of LVB, ruling out any intermolecular photoinduced process between the two guests despite sharing the same host. Ad-hoc devised Förster Resonance Energy Transfer experiments demonstrate this to be the result of the not close proximity of the two guests, which are confined in different compartments of the same polymeric host. The supramolecular complex is stable in a culture medium, and its biological activity has been evaluated against HEP-G2 hepatocarcinoma cell lines in the dark and under irradiation with visible green light, using LVB at a concentration well below the IC50. Comparative experiments performed using the polymeric host encapsulating the individual LVB and RD-NO components under the same experimental conditions show that the moderate cell mortality induced by the ternary complex in the dark increases significantly upon irradiation with visible green light, more likely as the result of synergism between the NO photogenerated and the chemotherapeutic.
Keywords: chemotherapeutic; combination therapy; cyclodextrin polymers; light; nitric oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kiyota N., Schlumberger M., Muro K., Ando Y., Takahashi S., Kawai Y., Wirth L., Robinson B., Sherman S., Suzuki T., et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 2015;106:1714–1721. doi: 10.1111/cas.12826. - DOI - PMC - PubMed
-
- Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., Baron A., Park J.W., Han G., Jassem J., et al. Levantinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

