The "Light Knife" for Gastric Cancer: Photodynamic Therapy
- PMID: 36678730
- PMCID: PMC9860630
- DOI: 10.3390/pharmaceutics15010101
The "Light Knife" for Gastric Cancer: Photodynamic Therapy
Abstract
Photodynamic therapy (PDT) has been used clinically to treat cancer for more than 40 years. Some solid tumors, including esophageal cancer, lung cancer, head and neck cancer, cholangiocarcinoma, and bladder cancer, have been approved for and managed with PDT in many countries globally. Notably, PDT for gastric cancer (GC) has been reported less and is not currently included in the clinical diagnosis and treatment guidelines. However, PDT is a potential new therapeutic modality used for the management of GC, and its outcomes and realization are more and more encouraging. PDT has a pernicious effect on tumors at the irradiation site and can play a role in rapid tumor shrinkage when GC is combined with cardiac and pyloric obstruction. Furthermore, because of its ability to activate the immune system, it still has a specific effect on systemic metastatic lesions, and the adverse reactions are mild. In this Review, we provide an overview of the current application progress of PDT for GC; systematically elaborate on its principle, mechanism, and the application of a new photosensitizer in GC; and focus on the efficacy of PDT in GC and the prospect of combined use with other therapeutic methods to provide a theoretical basis for clinical application.
Keywords: chemotherapy; combination therapy; gastric cancer; immunotherapy; photodynamic therapy; photosensitizer; target therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


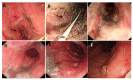

References
-
- World Health Organization . International Agency for Research on Cancer. GLOBOCAN 2020: Stomach Cancer Fact Sheet. WHO; Geneva, Switzerland: 2020.
-
- Bang Y.J., Van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., Lordick F., Ohtsu A., Omuro Y., Satoh T., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. - DOI - PubMed
-
- Wilke H., Muro K., Van Cutsem E., Oh S.C., Bodoky G., Shimada Y., Hironaka S., Sugimoto N., Lipatov O., Kim T.Y., et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. - DOI - PubMed
-
- Dank M., Zaluski J., Barone C., Valvere V., Yalcin S., Peschel C., Wenczl M., Goker E., Cisar L., Wang K., et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann. Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

