Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems
- PMID: 36678839
- PMCID: PMC9862045
- DOI: 10.3390/pharmaceutics15010210
Microfluidic Manipulation for Biomedical Applications in the Central and Peripheral Nervous Systems
Abstract
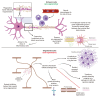
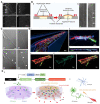
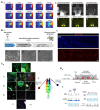
Physical injuries and neurodegenerative diseases often lead to irreversible damage to the organizational structure of the central nervous system (CNS) and peripheral nervous system (PNS), culminating in physiological malfunctions. Investigating these complex and diverse biological processes at the macro and micro levels will help to identify the cellular and molecular mechanisms associated with nerve degeneration and regeneration, thereby providing new options for the development of new therapeutic strategies for the functional recovery of the nervous system. Due to their distinct advantages, modern microfluidic platforms have significant potential for high-throughput cell and organoid cultures in vitro, the synthesis of a variety of tissue engineering scaffolds and drug carriers, and observing the delivery of drugs at the desired speed to the desired location in real time. In this review, we first introduce the types of nerve damage and the repair mechanisms of the CNS and PNS; then, we summarize the development of microfluidic platforms and their application in drug carriers. We also describe a variety of damage models, tissue engineering scaffolds, and drug carriers for nerve injury repair based on the application of microfluidic platforms. Finally, we discuss remaining challenges and future perspectives with regard to the promotion of nerve injury repair based on engineered microfluidic platform technology.
Keywords: damage models; drug-delivery system; microfluidic platforms; nerve injury repair; tissue engineering scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Advances in novel biomaterials combined with traditional Chinese medicine rehabilitation technology in treatment of peripheral nerve injury.Front Neurol. 2024 Jun 13;15:1421772. doi: 10.3389/fneur.2024.1421772. eCollection 2024. Front Neurol. 2024. PMID: 38938781 Free PMC article. Review.
-
Investigation of nerve injury through microfluidic devices.J R Soc Interface. 2013 Nov 13;11(90):20130676. doi: 10.1098/rsif.2013.0676. Print 2014 Jan 6. J R Soc Interface. 2013. PMID: 24227311 Free PMC article. Review.
-
Alginate-Based Hydrogels and Tubes, as Biological Macromolecule-Based Platforms for Peripheral Nerve Tissue Engineering: A Review.Ann Biomed Eng. 2022 Jun;50(6):628-653. doi: 10.1007/s10439-022-02955-8. Epub 2022 Apr 21. Ann Biomed Eng. 2022. PMID: 35446001 Review.
-
High-throughput screening approaches and combinatorial development of biomaterials using microfluidics.Acta Biomater. 2016 Apr 1;34:1-20. doi: 10.1016/j.actbio.2015.09.009. Epub 2015 Sep 8. Acta Biomater. 2016. PMID: 26361719 Review.
-
Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges.Crit Rev Biomed Eng. 2011;39(2):81-124. doi: 10.1615/critrevbiomedeng.v39.i2.20. Crit Rev Biomed Eng. 2011. PMID: 21488817 Review.
Cited by
-
Advances in novel biomaterials combined with traditional Chinese medicine rehabilitation technology in treatment of peripheral nerve injury.Front Neurol. 2024 Jun 13;15:1421772. doi: 10.3389/fneur.2024.1421772. eCollection 2024. Front Neurol. 2024. PMID: 38938781 Free PMC article. Review.
-
Photobiomodulation of Neurogenesis through the Enhancement of Stem Cell and Neural Progenitor Differentiation in the Central and Peripheral Nervous Systems.Int J Mol Sci. 2023 Oct 21;24(20):15427. doi: 10.3390/ijms242015427. Int J Mol Sci. 2023. PMID: 37895108 Free PMC article. Review.
-
Recent progresses in neural tissue engineering using topographic scaffolds.Am J Stem Cells. 2024 Feb 25;13(1):1-26. doi: 10.62347/WMDZ8890. eCollection 2024. Am J Stem Cells. 2024. PMID: 38505822 Free PMC article. Review.
-
Peripheral nerve injury repair by electrical stimulation combined with graphene-based scaffolds.Front Bioeng Biotechnol. 2024 Feb 28;12:1345163. doi: 10.3389/fbioe.2024.1345163. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38481574 Free PMC article. Review.
-
TPP-Based Microfluidic Chip Design and Fabrication Method for Optimized Nerve Cells Directed Growth.Cyborg Bionic Syst. 2024 May 9;5:0095. doi: 10.34133/cbsystems.0095. eCollection 2024. Cyborg Bionic Syst. 2024. PMID: 38725973 Free PMC article.
References
-
- Carvalho C.R., Reis R.L., Oliveira J.M. Fundamentals and Current Strategies for Peripheral Nerve Repair and Regeneration. Adv. Exp. Med. Biol. 2020;1249:173–201. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

