Rice Phytoalexins: Half a Century of Amazing Discoveries; Part I: Distribution, Biosynthesis, Chemical Synthesis, and Biological Activities
- PMID: 36678973
- PMCID: PMC9862927
- DOI: 10.3390/plants12020260
Rice Phytoalexins: Half a Century of Amazing Discoveries; Part I: Distribution, Biosynthesis, Chemical Synthesis, and Biological Activities
Abstract
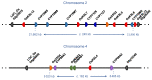
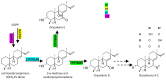
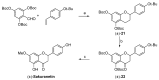
Cultivated rice is a staple food for more than half of the world's population, providing approximately 20% of the world's food energy needs. A broad spectrum of pathogenic microorganisms causes rice diseases leading to huge yield losses worldwide. Wild and cultivated rice species are known to possess a wide variety of antimicrobial secondary metabolites, known as phytoalexins, which are part of their active defense mechanisms. These compounds are biosynthesized transiently by rice in response to pathogens and certain abiotic stresses. Rice phytoalexins have been intensively studied for over half a century, both for their biological role and their potential application in agronomic and pharmaceutical fields. In recent decades, the growing interest of the research community, combined with advances in chemical, biological, and biomolecular investigation methods, has led to a notable acceleration in the growth of knowledge on rice phytoalexins. This review provides an overview of the knowledge gained in recent decades on the diversity, distribution, biosynthesis, chemical synthesis, and bioactivity of rice phytoalexins, with particular attention to the most recent advances in this research field.
Keywords: Oryza; momilactones; oryzalexins; phenilammides; phytoalexins; phytocassanes; rice; sakuranetin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
-
- Dai L.Y., Liu X.L., Xiao Y.H., Wang G.L. Recent advances in cloning and characterization of disease resistance genes in rice. J. Integr. Plant Biol. 2007;49:112–119. doi: 10.1111/j.1744-7909.2006.00413.x. - DOI
-
- Sparks A., Nelson A., Castilla N. Where rice pests and diseases do the most damage. Rice Today. 2012;11:27.
-
- Müller K.O., Meyer G., Klinkowski M. Physiologisch-genetische Untersuchungen über die Resistenz der Kartoffel gegenüber Phytophthora infestans. Naturwissenschaften. 1939;27:765–768. doi: 10.1007/BF01498120. - DOI
Publication types
LinkOut - more resources
Full Text Sources

