Influence of Nitrogen on Grapevine Susceptibility to Downy Mildew
- PMID: 36678977
- PMCID: PMC9867458
- DOI: 10.3390/plants12020263
Influence of Nitrogen on Grapevine Susceptibility to Downy Mildew
Abstract
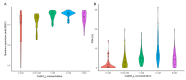
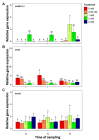
Downy mildew, caused by the obligate parasite Plasmopara viticola, is one of the most important threats to viticulture. The exploitation of resistant and susceptibility traits of grapevine is one of the most promising ways to increase the sustainability of disease management. Nitrogen (N) fertilization is known for influencing disease severity in the open field, but no information is available on its effect on plant-pathogen interaction. A previous RNAseq study showed that several genes of N metabolism are differentially regulated in grapevine upon P. viticola inoculation, and could be involved in susceptibility or resistance to the pathogen. The aim of this study was to evaluate if N fertilization influences: (i) the foliar leaf content and photosynthetic activity of the plant, (ii) P. viticola infectivity, and (iii) the expression of the candidate susceptibility/resistance genes. Results showed that N level positively correlated with P. viticola infectivity, confirming that particular attention should be taken in vineyard to the fertilization, but did not influence the expression of the candidate genes. Therefore, these genes are manipulated by the pathogen and can be exploited for developing new, environmentally friendly disease management tools, such as dsRNAs, to silence the susceptibility genes or breeding for resistance.
Keywords: Vitis vinifera; nitrogen fertilization; oomycete; plant pathogen interaction; resistance genes; susceptibility genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Conklin A., Stilwell T. World Food. John Wiley & Sons; Hoboken, NJ, USA: 2007.
-
- Huber D., Thompson I. Nitrogen and plant disease. In: Datnoff L., Elmer W., Huber D., editors. Mineral Nutrition and Plant Disease. APS Press; St. Paul, MN, USA: 2007. pp. 31–44.
-
- Verdenal T., Dienes-Nagy Á., Spangenberg J.E., Zufferey V., Spring J.-L., Viret O., Marin-Carbonne J., Van Leeuwen C. Understanding and managing nitrogen nutrition in grapevine: A review. OENO One. 2021;55:1–43. doi: 10.20870/oeno-one.2021.55.1.3866. - DOI
-
- Keller M. Deficit Irrigation and Vine Mineral Nutrition. Am. J. Enol. Vitic. 2005;56:267–283. doi: 10.5344/ajev.2005.56.3.267. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

