ATANN3 Is Involved in Extracellular ATP-Regulated Auxin Distribution in Arabidopsis thaliana Seedlings
- PMID: 36679043
- PMCID: PMC9867528
- DOI: 10.3390/plants12020330
ATANN3 Is Involved in Extracellular ATP-Regulated Auxin Distribution in Arabidopsis thaliana Seedlings
Abstract
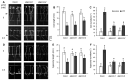
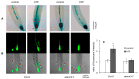
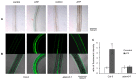
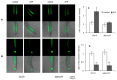
Extracellular ATP (eATP) plays multiple roles in plant growth and development, and stress responses. It has been revealed that eATP suppresses growth and alters the growth orientation of the root and hypocotyl of Arabidopsis thaliana by affecting auxin transport and localization in these organs. However, the mechanism of the eATP-stimulated auxin distribution remains elusive. Annexins are involved in multiple aspects of plant cellular metabolism, while their role in response to apoplastic signals remains unclear. Here, by using the loss-of-function mutations, we investigated the role of AtANN3 in the eATP-regulated root and hypocotyl growth. Firstly, the inhibitory effects of eATP on root and hypocotyl elongation were weakened or impaired in the AtANN3 null mutants (atann3-1 and atann3-2). Meanwhile, the distribution of DR5-GUS and DR5-GFP indicated that the eATP-induced asymmetric distribution of auxin in the root tips or hypocotyl cells occurred in wild-type control plants, while in atann3-1 mutant seedlings, it was not observed. Further, the eATP-induced asymmetric distribution of PIN2-GFP in root-tip cells or that of PIN3-GFP in hypocotyl cells was reduced in atann3-1 seedlings. Finally, the eATP-induced asymmetric distribution of cytoplasmic vesicles in root-tip cells was impaired in atann3-1 seedlings. Based on these results, we suggest that AtANN3 may be involved in eATP-regulated seedling growth by regulating the distribution of auxin and auxin transporters in vegetative organs.
Keywords: Arabidopsis thaliana; AtANN3; auxin; extracellular ATP (eATP); seedling growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

