Protein Changes in Shade and Sun Haberlea rhodopensis Leaves during Dehydration at Optimal and Low Temperatures
- PMID: 36679114
- PMCID: PMC9861795
- DOI: 10.3390/plants12020401
Protein Changes in Shade and Sun Haberlea rhodopensis Leaves during Dehydration at Optimal and Low Temperatures
Abstract
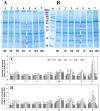
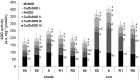
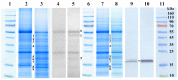
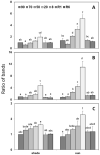
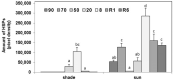
Haberlea rhodopensis is a unique resurrection plant of high phenotypic plasticity, colonizing both shady habitats and sun-exposed rock clefts. H. rhodopensis also survives freezing winter temperatures in temperate climates. Although survival in conditions of desiccation and survival in conditions of frost share high morphological and physiological similarities, proteomic changes lying behind these mechanisms are hardly studied. Thus, we aimed to reveal ecotype-level and temperature-dependent variations in the protective mechanisms by applying both targeted and untargeted proteomic approaches. Drought-induced desiccation enhanced superoxide dismutase (SOD) activity, but FeSOD and Cu/ZnSOD-III were significantly better triggered in sun plants. Desiccation resulted in the accumulation of enzymes involved in carbohydrate/phenylpropanoid metabolism (enolase, triosephosphate isomerase, UDP-D-apiose/UDP-D-xylose synthase 2, 81E8-like cytochrome P450 monooxygenase) and protective proteins such as vicinal oxygen chelate metalloenzyme superfamily and early light-induced proteins, dehydrins, and small heat shock proteins, the latter two typically being found in the latest phases of dehydration and being more pronounced in sun plants. Although low temperature and drought stress-induced desiccation trigger similar responses, the natural variation of these responses in shade and sun plants calls for attention to the pre-conditioning/priming effects that have high importance both in the desiccation responses and successful stress recovery.
Keywords: LC-MS/MS; drought stress; frost-induced desiccation; proteomics; resurrection plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Farrant J.M., Brandt W., Lindsey G. An overview of mechanisms of desiccation tolerance in selected angiosperm resurrection plants. Plant Stress. 2007;1:72–84. doi: 10.1002/9780470376881.ch3. - DOI
-
- Rakić T., Lazarević M., Jovanović Ž.S., Radović S., Siljak-Yakovlev S., Stevanović B., Stevanović V. Resurrection plants of the genus Ramonda: Prospective survival strategies-unlock further capacity of adaptation, or embark on the path of evolution? Front. Plant Sci. 2014;4:550. doi: 10.3389/fpls.2013.00550. - DOI - PMC - PubMed
-
- Gashi B., Babani F., Kongjika E. Chlorophyll fluorescence imaging of photosynthetic activity and pigment contents of the resurrection plants Ramonda serbica and Ramonda nathaliae during dehydration and rehydration. Physiol. Mol. Biol. Plants. 2013;19:333–341. doi: 10.1007/s12298-013-0175-5. - DOI - PMC - PubMed
-
- Fernández-Marín B., Nadal M., Gago J., Fernie A.R., López-Pozo M., Artetxe U., García-Plazaola J.I., Verhoeven A. Born to revive: Molecular and physiological mechanisms of double tolerance in a paleotropical and resurrection plant. New Phytol. 2020;226:741–759. doi: 10.1111/nph.16464. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

