Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity
- PMID: 36679188
- PMCID: PMC9861354
- DOI: 10.3390/polym15020307
Atmospheric Pressure Plasma Polymerisation of D-Limonene and Its Antimicrobial Activity
Abstract
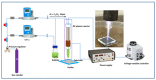
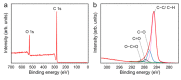
Antibacterial coating is necessary to prevent biofilm-forming bacteria from colonising medical tools causing infection and sepsis in patients. The recent coating strategies such as immobilisation of antimicrobial materials and low-pressure plasma polymerisation may require multiple processing steps involving a high-vacuum system and time-consuming process. Some of those have limited efficacy and durability. Here, we report a rapid and one-step atmospheric pressure plasma polymerisation (APPP) of D-limonene to produce nano-thin films with hydrophobic-like properties for antibacterial applications. The influence of plasma polymerisation time on the thickness, surface characteristic, and chemical composition of the plasma-polymerised films was systematically investigated. Results showed that the nano-thin films deposited at 1 min on glass substrate are optically transparent and homogenous, with a thickness of 44.3 ± 4.8 nm, a smooth surface with an average roughness of 0.23 ± 0.02 nm. For its antimicrobial activity, the biofilm assay evaluation revealed a significant 94% decrease in the number of Escherichia coli (E. coli) compared to the control sample. More importantly, the resultant nano-thin films exhibited a potent bactericidal effect that can distort and rupture the membrane of the treated bacteria. These findings provide important insights into the development of bacteria-resistant and biocompatible coatings on the arbitrary substrate in a straightforward and cost-effective route at atmospheric pressure.
Keywords: ASTM E2149; D-limonene; E. coli bacteria; antimicrobial coating; atmospheric pressure; plasma polymerisation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Plasma polymerized carvone as an antibacterial and biocompatible coating.Mater Sci Eng C Mater Biol Appl. 2016 Nov 1;68:861-871. doi: 10.1016/j.msec.2016.07.040. Epub 2016 Jul 18. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 27524089
-
Retention of Antibacterial Activity in Geranium Plasma Polymer Thin Films.Nanomaterials (Basel). 2017 Sep 13;7(9):270. doi: 10.3390/nano7090270. Nanomaterials (Basel). 2017. PMID: 28902134 Free PMC article.
-
Antibacterial activity studies of plasma polymerised cineole films.J Mater Chem B. 2014 Aug 21;2(31):4993-5002. doi: 10.1039/c4tb00633j. Epub 2014 Jun 11. J Mater Chem B. 2014. PMID: 32261832
-
Hydrophobic and superhydrophobic surfaces fabricated using atmospheric pressure cold plasma technology: A review.Adv Colloid Interface Sci. 2018 Apr;254:1-21. doi: 10.1016/j.cis.2018.03.009. Epub 2018 Mar 29. Adv Colloid Interface Sci. 2018. PMID: 29636183 Review.
-
Plasma surface modification strategies for the preparation of antibacterial biomaterials: A review of the recent literature.Mater Sci Eng C Mater Biol Appl. 2021 Dec;131:112474. doi: 10.1016/j.msec.2021.112474. Epub 2021 Oct 13. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34857260 Review.
Cited by
-
Plasma-Polymerised Antibacterial Coating of Ovine Tendon Collagen Type I (OTC) Crosslinked with Genipin (GNP) and Dehydrothermal-Crosslinked (DHT) as a Cutaneous Substitute for Wound Healing.Materials (Basel). 2023 Mar 29;16(7):2739. doi: 10.3390/ma16072739. Materials (Basel). 2023. PMID: 37049037 Free PMC article.
-
Role of Plasma in Catalyst Preparation and Modification for Oxygen Evolution Reaction.Precis Chem. 2024 Dec 12;3(3):110-127. doi: 10.1021/prechem.4c00075. eCollection 2025 Mar 24. Precis Chem. 2024. PMID: 40151810 Free PMC article. Review.
-
Zircon-Type CaCrO4 Chromite Nanoparticles: Synthesis, Characterization, and Photocatalytic Application for Sunlight-Induced Degradation of Rhodamine B.ACS Omega. 2023 Aug 7;8(33):30095-30108. doi: 10.1021/acsomega.3c02457. eCollection 2023 Aug 22. ACS Omega. 2023. PMID: 37636959 Free PMC article.
-
Identification of prochlorperazine dimaleate as a Sortase A inhibitor from FDA libraries for MRSA infection treatment.RSC Adv. 2025 Jun 25;15(27):21666-21677. doi: 10.1039/d5ra01781e. eCollection 2025 Jun 23. RSC Adv. 2025. PMID: 40567479 Free PMC article.
References
-
- Barros C.H., Casey E. A Review of Nanomaterials and Technologies for Enhancing the Antibiofilm Activity of Natural Products and Phytochemicals. ACS Appl. Nano Mater. 2020;3:8537–8556. doi: 10.1021/acsanm.0c01586. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

