Non-Destructive Direct Pericarp Thickness Measurement of Sorghum Kernels Using Extended-Focus Optical Coherence Microscopy
- PMID: 36679502
- PMCID: PMC9865951
- DOI: 10.3390/s23020707
Non-Destructive Direct Pericarp Thickness Measurement of Sorghum Kernels Using Extended-Focus Optical Coherence Microscopy
Abstract
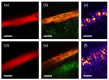
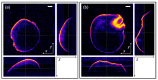
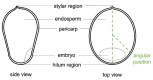
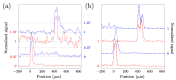
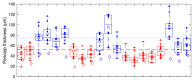
Non-destructive measurements of internal morphological structures in plant materials such as seeds are of high interest in agricultural research. The estimation of pericarp thickness is important to understand the grain quality and storage stability of seeds and can play a crucial role in improving crop yield. In this study, we demonstrate the applicability of fiber-based Bessel beam Fourier domain (FD) optical coherence microscopy (OCM) with a nearly constant high lateral resolution maintained at over ~400 µm for direct non-invasive measurement of the pericarp thickness of two different sorghum genotypes. Whereas measurements based on axial profiles need additional knowledge of the pericarp refractive index, en-face views allow for direct distance measurements. We directly determine pericarp thickness from lateral sections with a 3 µm resolution by taking the width of the signal corresponding to the pericarp at the 1/e threshold. These measurements enable differentiation of the two genotypes with 100% accuracy. We find that trading image resolution for acquisition speed and view size reduces the classification accuracy. Average pericarp thicknesses of 74 µm (thick phenotype) and 43 µm (thin phenotype) are obtained from high-resolution lateral sections, and are in good agreement with previously reported measurements of the same genotypes. Extracting the morphological features of plant seeds using Bessel beam FD-OCM is expected to provide valuable information to the food processing industry and plant breeding programs.
Keywords: Bessel beam; Fourier domain optical coherence microscopy; higher-order-mode fiber; long-period grating; pericarp; phenotyping; sorghum; spatial resolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Smith C.W., Frederiksen R.A. Sorghum: Origin, History, Technology, and Production. Volume 2 John Wiley & Sons; New York, NY, USA: 2000.
-
- FAOSTAT Food and Agriculure Organization of United Nations. [(accessed on 22 December 2022)]. Available online: https://www.fao.org/faostat/en/#home.
-
- Hoseney R.C., Davis A.B., Harbers L.H. Pericarp and endosperm structure of sorghum shown by scanning electron microscopy. Cereal Chem. 1974;51:552–558.
-
- Earp C.F., McDonough C.M., Rooney L.W. Microscopy of pericarp development in the caryopsis of Sorghum bicolor (L.) Moench. J. Cereal Sci. 2004;39:21–27. doi: 10.1016/S0733-5210(03)00060-2. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

