Current Technology Developments Can Improve the Quality of Research and Level of Evidence for Rehabilitation Interventions: A Narrative Review
- PMID: 36679672
- PMCID: PMC9866361
- DOI: 10.3390/s23020875
Current Technology Developments Can Improve the Quality of Research and Level of Evidence for Rehabilitation Interventions: A Narrative Review
Abstract
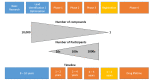
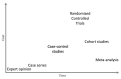
The current important limitations to the implementation of Evidence-Based Practice (EBP) in the rehabilitation field are related to the validation process of interventions. Indeed, most of the strict guidelines that have been developed for the validation of new drugs (i.e., double or triple blinded, strict control of the doses and intensity) cannot-or can only partially-be applied in rehabilitation. Well-powered, high-quality randomized controlled trials are more difficult to organize in rehabilitation (e.g., longer duration of the intervention in rehabilitation, more difficult to standardize the intervention compared to drug validation studies, limited funding since not sponsored by big pharma companies), which reduces the possibility of conducting systematic reviews and meta-analyses, as currently high levels of evidence are sparse. The current limitations of EBP in rehabilitation are presented in this narrative review, and innovative solutions are suggested, such as technology-supported rehabilitation systems, continuous assessment, pragmatic trials, rehabilitation treatment specification systems, and advanced statistical methods, to tackle the current limitations. The development and implementation of new technologies can increase the quality of research and the level of evidence supporting rehabilitation, provided some adaptations are made to our research methodology.
Keywords: methods; new technology; rehabilitation; study design; validation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Feinstein A.R. Clinical Judgment. Williams & Wilkins; Philadelphia, PA, USA: 1967.
-
- Cochrane A.L. Effectiveness & Efficiency: Random Reflections on Health Services. RSM Books; London, UK: 1999. New.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

