A Plant-Produced Porcine Parvovirus 1-82 VP2 Subunit Vaccine Protects Pregnant Sows against Challenge with a Genetically Heterologous PPV1 Strain
- PMID: 36679898
- PMCID: PMC9867127
- DOI: 10.3390/vaccines11010054
A Plant-Produced Porcine Parvovirus 1-82 VP2 Subunit Vaccine Protects Pregnant Sows against Challenge with a Genetically Heterologous PPV1 Strain
Abstract
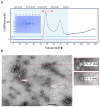
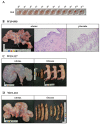
Porcine parvovirus (PPV) causes reproductive failure in sows, and vaccination remains the most effective means of preventing infection. The NADL-2 strain has been used as a vaccine for ~50 years; however, it does not protect animals against genetically heterologous PPV strains. Thus, new effective and safe vaccines are needed. In this study, we aimed to identify novel PPV1 strains, and to develop PPV1 subunit vaccines. We isolated and sequenced PPV1 VP2 genes from 926 pigs and identified ten PPV1 strains (belonging to Groups C, D and E). We selected the Group D PPV1-82 strain as a vaccine candidate because it was close to the highly pathogenic 27a strain. The PPV1-82 VP2 protein was produced in Nicotiana benthamiana. It formed virus-like particles and exhibited a 211 agglutination value. The PPV1-190313 strain (Group E), isolated from an aborted fetus, was used as the challenging strain because it was pathogenic. The unvaccinated sow miscarried at 8 days postchallenge, and mummified fetuses were all PPV1-positive. By contrast, pregnant sows vaccinated with PPV1-82 VP2 had 9-11 Log2 antibody titers and produced normal fetuses after PPV1-190313 challenge. These results suggest the PPV1-82 VP2 subunit vaccine protects pregnant sows against a genetically heterologous PPV1 strain by inducing neutralizing antibodies.
Keywords: cross reactivation; plant-produced subunit vaccine; porcine parvovirus 1; viral protein 2; virus-like particle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Cartwright S.F., Huck R. Viruses isolated in association with herd infertility absortions and stillbirths in pigs. Vet. Rec. 1967;81:196.
Grants and funding
LinkOut - more resources
Full Text Sources

