Immunogenicity of BNT162b2, BBIBP-CorV, Gam-COVID-Vac and ChAdOx1 nCoV-19 Vaccines Six Months after the Second Dose: A Longitudinal Prospective Study
- PMID: 36679901
- PMCID: PMC9865554
- DOI: 10.3390/vaccines11010056
Immunogenicity of BNT162b2, BBIBP-CorV, Gam-COVID-Vac and ChAdOx1 nCoV-19 Vaccines Six Months after the Second Dose: A Longitudinal Prospective Study
Abstract
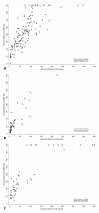
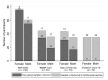
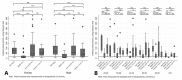
Many available SARS-CoV-2 vaccines demonstrated good humoral response, but studies directly comparing their immunogenicity in the general population are lacking. We evaluated the medium−term kinetics of anti-S SARS-CoV-2 antibodies (Abs) at one and six months after the second dose of BNT162b2, BBIBP-CorV, and Gam-COVID-Vac. Immunogenicity at six months was directly compared between BNT162b2, BBIBP-CorV, Gam-COVID-Vac, and ChAdOx1 nCoV-19. Participants ≥ 20 years old from Novi Sad, Serbia, without prior SARS-CoV-2 infection, were included. Anti S1/S2 IgG antibodies were measured using quantitative LIAISON SARS-CoV-2 assay. A total of 368 participants were included: 231 (62.77%) had sera collected at two time points. Two doses of BNT162b2 were received by 37.50% of participants, followed by BBIBP-CorV (22.01%), Gam-COVID-Vac (21.47%), and ChAdOx1 nCoV-19 (19.02%). Mean Ab levels at the 28th day and 6 months were 216.55 (SD = 105.73) AU/mL and 75.68 (SD = 57.30) for BNT162b2, 194.38 (SD = 140.24) and 90.53 (SD = 111.30) for Gam-COVID-Vac, and 72.74 (SD = 80.04) and 24.43 (SD = 38.43) for BBIBP-CorV group (p < 0.01, between two time points across all three groups), with a significant difference between women and men (p < 0.01, for both sexes). At the sixth month post-vaccination, the highest mean Ab level was detected in Gam-COVID-Vac group (91.28 AU/mL, SD = 95.96), followed by BNT162b2 (85.25 AU/mL, SD = 60.02), ChAdOx1 nCoV-19 (64.22 AU/mL, SD = 65.30), and BBIBP-CorV (25.26 AU/mL, SD = 36.92) (p < 0.01). Anti-spike IgG persistence was demonstrated six months post-vaccination with a significant decline in Ab levels. These results suggest a lower protection against SARS-CoV-2 over time. Our findings support the introduction of additional (booster) doses.
Keywords: BBIBP-CorV; BNT162b2; COVID-19; ChAdOx1 nCoV-19; Gam-COVID-Vac; immunogenicity; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

