Evaluation of Two Adjuvant Formulations for an Inactivated Yellow Fever 17DD Vaccine Candidate in Mice
- PMID: 36679918
- PMCID: PMC9865672
- DOI: 10.3390/vaccines11010073
Evaluation of Two Adjuvant Formulations for an Inactivated Yellow Fever 17DD Vaccine Candidate in Mice
Abstract
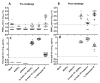
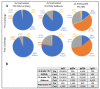
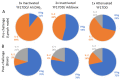
The attenuated yellow fever (YF) vaccine is one of the most successful vaccines ever developed. After a single dose administration YF vaccine can induce balanced Th1/Th2 immune responses and long-lasting neutralizing antibodies. These attributes endorsed it as a model of how to properly stimulate the innate response to target protective immune responses. Despite their longstanding success, attenuated YF vaccines can cause rare fatal adverse events and are contraindicated for persons with immunosuppression, egg allergy and age < 6 months and >60 years. These drawbacks have encouraged the development of a non-live vaccine. The aim of the present study is to characterize and compare the immunological profile of two adjuvant formulations of an inactivated YF 17DD vaccine candidate. Inactivated YF vaccine formulations based on alum (Al(OH)3) or squalene (AddaVax®) were investigated by immunization of C57BL/6 mice in 3-dose or 2-dose schedules, respectively, and compared with a single dose of attenuated YF virus 17DD. Sera were analyzed by ELISA and Plaque Reduction Neutralization Test (PRNT) for detection of total IgG and neutralizing antibodies against YF virus. In addition, splenocytes were collected to evaluate cellular responses by ELISpot. Both inactivated formulations were able to induce high titers of IgG against YF, although neutralizing antibodies levels were borderline on pre-challenge samples. Analysis of IgG subtypes revealed a predominance of IgG2a associated with improved neutralizing capacity in animals immunized with the attenuated YF vaccine, and a predominance of IgG1 in groups immunized with experimental non-live formulations (alum and AddaVax®). After intracerebral (IC) challenge, attenuated and inactivated vaccine formulations showed an increase in neutralizing antibodies. The AddaVax®-based inactivated vaccine and the attenuated vaccine achieved 100% protection, and alum-based equivalent formulation achieved 70% protection.
Keywords: adjuvants; immunogenicity; protective efficacy; yellow fever inactivated vaccine.
Conflict of interest statement
The authors declare that they have no conflicts of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- De Oliveira Figueiredo P., Stoffella-Dutra A.G., Barbosa Costa G., Silva de Oliveira J., Dourado Amaral C., Duarte Santos J., Soares Rocha K.L., Araújo Júnior J.P., Lacerda Nogueira M., Zazá Borges M.A., et al. Re-Emergence of Yellow Fever in Brazil during 2016–2019: Challenges, Lessons Learned, and Perspectives. Viruses. 2020;12:1233. doi: 10.3390/v12111233. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

