Differences in BNT126b2 and ChAdOx1 Homologous Vaccination Antibody Response among Teachers in Poznan, Poland
- PMID: 36679962
- PMCID: PMC9862687
- DOI: 10.3390/vaccines11010118
Differences in BNT126b2 and ChAdOx1 Homologous Vaccination Antibody Response among Teachers in Poznan, Poland
Abstract
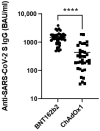
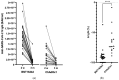
Children are among the best vectors to spread respiratory viruses, including emerging variants of SARS-CoV-2 due to the asymptomatic or relatively mild course of infection and simultaneously high titres of pathogens in the respiratory tract. Therefore, individuals who have constant contact with children, e.g., teachers should be vaccinated against COVID-19 as essential workers within the first phases of a vaccination campaign. In Poland, primary and secondary school teachers were vaccinated with ChAdOx1 from February 2021 with a three month interval between the two doses, while lecturers at medical universities, who are simultaneously healthcare workers, received the BNT126b2 vaccine from December 2020 with three weeks between the first and second doses. The aim of this study was to compare the antibody responses at two weeks and three months after vaccination and to estimate the vaccine effectiveness against COVID-19 among infection-naïve teachers vaccinated with mRNA and a vector vaccine. We found that the anti-SARS-CoV-2 spike protein antibodies were significantly higher among the lecturers but antibody waning was slower among the schoolteachers. However, those vaccinated with ChAdOx1 complained significantly more often of vaccine side effects. In addition, during the three months after the second vaccine dose no study participants were infected with SARS-CoV-2. The BNT126b2 vaccine gave higher antibody titres in comparison with ChAdOx1 but protection against COVID-19 in both cases was similar. Moreover, we did not find any anti-SARS-CoV-2 nucleoprotein antibodies at two weeks as well as at three months after vaccination among the study participants, which shows a very high vaccine effectiveness in the occupational group with a high SARS-CoV-2-infection risk.
Keywords: Poland; SARS-CoV-2; antibodies; seroprevalence; teachers; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- WHO Coronavirus (COVID-19) Dashboard. [(accessed on 14 November 2022)]. Available online: https://covid19.who.int.
-
- COVID-19 Vaccine Tracker and Landscape. [(accessed on 14 November 2022)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

