Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population
- PMID: 36679980
- PMCID: PMC9861214
- DOI: 10.3390/vaccines11010135
Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population
Abstract
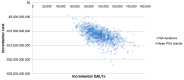
This study evaluated the clinical and economic impact of routine pediatric vaccination with the 15-valent pneumococcal conjugate vaccine (PCV15, V114) compared with the 13-valent PCV (PCV13) from a societal perspective in the United States (US). A Markov decision-analytic model was constructed to estimate the outcomes for the entire US population over a 100-year time horizon. The model estimated the impact of V114 versus PCV13 on pneumococcal disease (PD) incidence, post meningitis sequalae, and deaths, taking herd immunity effects into account. V114 effectiveness was extrapolated from the observed PCV13 data and PCV7 clinical trials. Costs (2021$) included vaccine acquisition and administration costs, direct medical costs for PD treatment, direct non-medical costs, and indirect costs, and were discounted at 3% per year. In the base case, V114 prevented 185,711 additional invasive pneumococcal disease, 987,727 all-cause pneumonia, and 11.2 million pneumococcal acute otitis media cases, compared with PCV13. This led to expected gains of 90,026 life years and 96,056 quality-adjusted life years with a total saving of $10.8 billion. Sensitivity analysis showed consistent results over plausible values of key model inputs and assumptions. The findings suggest that V114 is a cost-saving option compared to PCV13 in the routine pediatric vaccination program.
Keywords: PCV15; V114; cost-effectiveness; pediatric vaccination; pneumococcal diseases; public health impact.
Conflict of interest statement
Min Huang, Jessica Weaver, Kwame Owusu-Edusei and Elamin Elbasha are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Tianyan Hu is a former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Figures

Similar articles
-
Cost-Effectiveness Analysis of Pneumococcal Vaccines in the Pediatric Population: A Systematic Review.Healthcare (Basel). 2024 Sep 29;12(19):1950. doi: 10.3390/healthcare12191950. Healthcare (Basel). 2024. PMID: 39408130 Free PMC article. Review.
-
Cost-effectiveness of PCV20 to Prevent Pneumococcal Disease in the Pediatric Population: A German Societal Perspective Analysis.Infect Dis Ther. 2024 Jun;13(6):1333-1358. doi: 10.1007/s40121-024-00977-4. Epub 2024 May 11. Infect Dis Ther. 2024. PMID: 38733494 Free PMC article.
-
Cost-effectiveness analysis of pediatric immunization program with 15-valent pneumococcal conjugate vaccine in Japan.J Med Econ. 2023 Jan-Dec;26(1):1034-1046. doi: 10.1080/13696998.2023.2245291. J Med Econ. 2023. PMID: 37555281
-
Health and economic burden of invasive pneumococcal disease associated with 15-valent pneumococcal conjugate vaccine serotypes in children across eight European countries.J Med Econ. 2021 Jan-Dec;24(1):1098-1107. doi: 10.1080/13696998.2021.1970975. J Med Econ. 2021. PMID: 34461796
-
PCV15, a pneumococcal conjugate vaccine, for the prevention of invasive pneumococcal disease in infants and children.Expert Rev Vaccines. 2024 Jan-Dec;23(1):137-147. doi: 10.1080/14760584.2023.2294153. Epub 2023 Dec 20. Expert Rev Vaccines. 2024. PMID: 38111990 Review.
Cited by
-
Burden of invasive pneumococcal disease, non-invasive all-cause pneumonia, and acute otitis media in hospitalized US children: a retrospective multi-center study from 2015 to 2020.BMC Health Serv Res. 2024 Dec 18;24(1):1574. doi: 10.1186/s12913-024-11898-w. BMC Health Serv Res. 2024. PMID: 39696329 Free PMC article.
-
Cost-Effectiveness Analysis of Routine Childhood Immunization with 20-Valent versus 15-Valent Pneumococcal Conjugate Vaccines in Germany.Vaccines (Basel). 2024 Sep 12;12(9):1045. doi: 10.3390/vaccines12091045. Vaccines (Basel). 2024. PMID: 39340075 Free PMC article.
-
Cost-effectiveness analysis of routine pediatric vaccination of 15-valent pneumococcal conjugate vaccine in South Korea.Hum Vaccin Immunother. 2025 Dec;21(1):2515650. doi: 10.1080/21645515.2025.2515650. Epub 2025 Jun 27. Hum Vaccin Immunother. 2025. PMID: 40576170 Free PMC article.
-
Cost-Effectiveness Analysis of Pneumococcal Vaccines in the Pediatric Population: A Systematic Review.Healthcare (Basel). 2024 Sep 29;12(19):1950. doi: 10.3390/healthcare12191950. Healthcare (Basel). 2024. PMID: 39408130 Free PMC article. Review.
-
Estimating the Cost-Effectiveness of Switching to Higher-Valency Pediatric Pneumococcal Conjugate Vaccines in the United Kingdom.Vaccines (Basel). 2023 Jun 28;11(7):1168. doi: 10.3390/vaccines11071168. Vaccines (Basel). 2023. PMID: 37514984 Free PMC article.
References
-
- Advisory Committee on Immunization Practices Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49:1–35. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

