Maturation of Aluminium Adsorbed Antigens Contributes to the Creation of Homogeneous Vaccine Formulations
- PMID: 36680000
- PMCID: PMC9862877
- DOI: 10.3390/vaccines11010155
Maturation of Aluminium Adsorbed Antigens Contributes to the Creation of Homogeneous Vaccine Formulations
Abstract
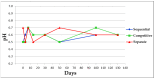
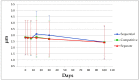
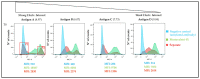
Although aluminium-based vaccines have been used for almost over a century, their mechanism of action remains unclear. It is established that antigen adsorption to the adjuvant facilitates delivery of the antigen to immune cells at the injection site. To further increase our understanding of aluminium-based vaccines, it is important to gain additional insights on the interactions between the aluminium and antigens, including antigen distribution over the adjuvant particles. Immuno-assays can further help in this regard. In this paper, we evaluated how established formulation strategies (i.e., sequential, competitive, and separate antigen addition) applied to four different antigens and aluminium oxyhydroxide, lead to formulation changes over time. Results showed that all formulation samples were stable, and that no significant changes were observed in terms of physical-chemical properties. Antigen distribution across the bulk aluminium population, however, did show a maturation effect, with some initial dependence on the formulation approach and the antigen adsorption strength. Sequential and competitive approaches displayed similar results in terms of the homogeneity of antigen distribution across aluminium particles, while separately adsorbed antigens were initially more highly poly-dispersed. Nevertheless, the formulation sample prepared via separate adsorption also reached homogeneity according to each antigen adsorption strength. This study indicated that antigen distribution across aluminium particles is a dynamic feature that evolves over time, which is initially influenced by the formulation approach and the specific adsorption strength, but ultimately leads to homogeneous formulations.
Keywords: adsorption; ageing; aluminium hydroxide; compounding strategy; distribution; homogeneity; maturation; populations.
Conflict of interest statement
This work was sponsored by GlaxoSmithKline Biologicals SA. The authors D.L., A.M., C.P., M.M. and D.T.O’H. are currently employed by the GSK group of companies. Please note that the authors C.S. and B.B. were employed by GSK group of companies at the time of the study, currently they are not longer employed by the GSK group of companies.
Figures






Similar articles
-
The in vitro displacement of adsorbed model antigens from aluminium-containing adjuvants by interstitial proteins.Vaccine. 1999 Jul 16;17(22):2873-81. doi: 10.1016/s0264-410x(99)00126-7. Vaccine. 1999. PMID: 10438058
-
Nanoalum adjuvanted vaccines: small details make a big difference.Semin Immunol. 2021 Aug;56:101544. doi: 10.1016/j.smim.2021.101544. Epub 2021 Dec 9. Semin Immunol. 2021. PMID: 34895823 Review.
-
Structural perturbation of diphtheria toxoid upon adsorption to aluminium hydroxide adjuvant.Vaccine. 2012 Nov 6;30(48):6783-8. doi: 10.1016/j.vaccine.2012.09.020. Epub 2012 Sep 18. Vaccine. 2012. PMID: 23000120
-
Quantitative Analysis of Vaccine Antigen Adsorption to Aluminum Adjuvant Using an Automated High-Throughput Method.PDA J Pharm Sci Technol. 2018 Mar-Apr;72(2):149-162. doi: 10.5731/pdajpst.2017.008250. Epub 2018 Jan 17. PDA J Pharm Sci Technol. 2018. PMID: 29343619
-
Reduced-antigen, combined diphtheria, tetanus and acellular pertussis vaccine, adsorbed (Boostrix®): a review of its properties and use as a single-dose booster immunization.Drugs. 2012 Sep 10;72(13):1765-91. doi: 10.2165/11209630-000000000-00000. Drugs. 2012. PMID: 22931522 Review.
Cited by
-
Analytical Insights into Protein-Alum Interactions and Their Impact on Conformational Epitope.Pharmaceutics. 2024 Mar 19;16(3):420. doi: 10.3390/pharmaceutics16030420. Pharmaceutics. 2024. PMID: 38543314 Free PMC article.
-
Nanoalum Formulations Containing Aluminum Hydroxide and CpG 1018TM Adjuvants: The Effect on Stability and Immunogenicity of a Recombinant SARS-CoV-2 RBD Antigen.Vaccines (Basel). 2023 May 26;11(6):1030. doi: 10.3390/vaccines11061030. Vaccines (Basel). 2023. PMID: 37376419 Free PMC article.
-
Formulation development and comparability studies with an aluminum-salt adjuvanted SARS-CoV-2 Spike ferritin nanoparticle vaccine antigen produced from two different cell lines.bioRxiv [Preprint]. 2023 Apr 4:2023.04.03.535447. doi: 10.1101/2023.04.03.535447. bioRxiv. 2023. Update in: Vaccine. 2023 Oct 20;41(44):6502-6513. doi: 10.1016/j.vaccine.2023.08.037. PMID: 37066156 Free PMC article. Updated. Preprint.
-
New High-Throughput Method for Aluminum Content Determination in Vaccine Formulations.Vaccines (Basel). 2025 Jan 22;13(2):105. doi: 10.3390/vaccines13020105. Vaccines (Basel). 2025. PMID: 40006652 Free PMC article.
-
Formulation development and comparability studies with an aluminum-salt adjuvanted SARS-CoV-2 spike ferritin nanoparticle vaccine antigen produced from two different cell lines.Vaccine. 2023 Oct 20;41(44):6502-6513. doi: 10.1016/j.vaccine.2023.08.037. Epub 2023 Aug 22. Vaccine. 2023. PMID: 37620203 Free PMC article.
References
LinkOut - more resources
Full Text Sources

