S Trimer Derived from SARS-CoV-2 B.1.351 and B.1.618 Induced Effective Immune Response against Multiple SARS-CoV-2 Variants
- PMID: 36680037
- PMCID: PMC9863711
- DOI: 10.3390/vaccines11010193
S Trimer Derived from SARS-CoV-2 B.1.351 and B.1.618 Induced Effective Immune Response against Multiple SARS-CoV-2 Variants
Abstract
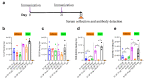
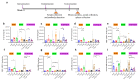
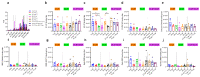
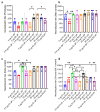
The spread of SARS-CoV-2 and its variants leads to a heavy burden on healthcare and the global economy, highlighting the need for developing vaccines that induce broad immunity against coronavirus. Here, we explored the immunogenicity of monovalent or bivalent spike (S) trimer subunit vaccines derived from SARS-CoV-2 B.1.351 (S1-2P) or/and B.1. 618 (S2-2P) in Balb/c mice. Both S1-2P and S2-2P elicited anti-spike antibody responses, and alum adjuvant induced higher levels of antibodies than Addavax adjuvant. The dose responses of the vaccines on immunogenicity were evaluated in vivo. A low dose of 5 μg monovalent recombinant protein or 2.5 μg bivalent vaccine triggered high-titer antibodies that showed cross-activity to Beta, Delta, and Gamma RBD in mice. The third immunization dose could boost (1.1 to 40.6 times) high levels of cross-binding antibodies and elicit high titers of neutralizing antibodies (64 to 1024) prototype, Beta, Delta, and Omicron variants. Furthermore, the vaccines were able to provoke a Th1-biased cellular immune response. Significantly, at the same antigen dose, S1-2P immune sera induced stronger broadly neutralizing antibodies against prototype, Beta, Delta, and Omicron variants compared to that induced by S2-2P. At the same time, the low dose of bivalent vaccine containing S2-2P and S1-2P (2.5 μg for each antigen) significantly improved the cross-neutralizing antibody responses. In conclusion, our results showed that monovalent S1-2P subunit vaccine or bivalent vaccine (S1-2P and S2-2P) induced potent humoral and cellular responses against multiple SARS-CoV-2 variants and provided valuable information for the development of recombinant protein-based SARS-CoV-2 vaccines that protect against emerging SARS-CoV-2 variants.
Keywords: SARS-CoV-2; beta variant; immune response; spike; trimer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Elicitation of Broadly Neutralizing Antibodies against B.1.1.7, B.1.351, and B.1.617.1 SARS-CoV-2 Variants by Three Prototype Strain-Derived Recombinant Protein Vaccines.Viruses. 2021 Jul 22;13(8):1421. doi: 10.3390/v13081421. Viruses. 2021. PMID: 34452287 Free PMC article.
-
A Glycosylated RBD Protein Induces Enhanced Neutralizing Antibodies against Omicron and Other Variants with Improved Protection against SARS-CoV-2 Infection.J Virol. 2022 Sep 14;96(17):e0011822. doi: 10.1128/jvi.00118-22. Epub 2022 Aug 16. J Virol. 2022. PMID: 35972290 Free PMC article.
-
Human Fc-Conjugated Receptor Binding Domain-Based Recombinant Subunit Vaccines with Short Linker Induce Potent Neutralizing Antibodies against Multiple SARS-CoV-2 Variants.Vaccines (Basel). 2022 Sep 8;10(9):1502. doi: 10.3390/vaccines10091502. Vaccines (Basel). 2022. PMID: 36146579 Free PMC article.
-
Significance of Conserved Regions in Coronavirus Spike Protein for Developing a Novel Vaccine against SARS-CoV-2 Infection.Vaccines (Basel). 2023 Feb 24;11(3):545. doi: 10.3390/vaccines11030545. Vaccines (Basel). 2023. PMID: 36992129 Free PMC article. Review.
-
Molecular and Clinical Investigation of COVID-19: From Pathogenesis and Immune Responses to Novel Diagnosis and Treatment.Front Mol Biosci. 2022 May 19;9:770775. doi: 10.3389/fmolb.2022.770775. eCollection 2022. Front Mol Biosci. 2022. PMID: 35664675 Free PMC article. Review.
Cited by
-
SARS-CoV-2 spike-FLIPr fusion protein plus lipidated FLIPr protects against various SARS-CoV-2 variants in hamsters.J Virol. 2024 Feb 20;98(2):e0154623. doi: 10.1128/jvi.01546-23. Epub 2024 Feb 1. J Virol. 2024. PMID: 38299865 Free PMC article.
-
SARS-CoV-2 variant survey: Comparison of RT-PCR screening with TGS and variant distribution across two divisions of Bangladesh.PLoS One. 2024 Oct 17;19(10):e0311993. doi: 10.1371/journal.pone.0311993. eCollection 2024. PLoS One. 2024. PMID: 39418288 Free PMC article.
References
-
- Chen L.L., Lu L., Choi C.Y.K., Cai J.P., Tsoi H.W., Chu A.W.-H., Ip J.D., Chan W.-M., Zhang R.R., Zhang X., et al. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variant-Associated Receptor Binding Domain (RBD) Mutations on the Susceptibility to Serum Antibodies Elicited by Coronavirus Disease 2019 (COVID-19) Infection or Vaccination. Clin. Infect. Dis. 2022;74:1623–1630. doi: 10.1093/cid/ciab656. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

