A Glimpse on the Evolution of RNA Viruses: Implications and Lessons from SARS-CoV-2
- PMID: 36680042
- PMCID: PMC9866536
- DOI: 10.3390/v15010001
A Glimpse on the Evolution of RNA Viruses: Implications and Lessons from SARS-CoV-2
Abstract
RNA viruses are characterised by extremely high genetic variability due to fast replication, large population size, low fidelity, and (usually) a lack of proofreading mechanisms of RNA polymerases leading to high mutation rates. Furthermore, viral recombination and reassortment may act as a significant evolutionary force among viruses contributing to greater genetic diversity than obtainable by mutation alone. The above-mentioned properties allow for the rapid evolution of RNA viruses, which may result in difficulties in viral eradication, changes in virulence and pathogenicity, and lead to events such as cross-species transmissions, which are matters of great interest in the light of current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemics. In this review, we aim to explore the molecular mechanisms of the variability of viral RNA genomes, emphasising the evolutionary trajectory of SARS-CoV-2 and its variants. Furthermore, the causes and consequences of coronavirus variation are explored, along with theories on the origin of human coronaviruses and features of emergent RNA viruses in general. Finally, we summarise the current knowledge on the circulating variants of concern and highlight the many unknowns regarding SARS-CoV-2 pathogenesis.
Keywords: RNA virus; SARS-CoV-2; coronavirus; evolution; mutation; quasispecies; recombination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
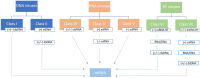
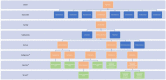

References
-
- Callanan J., Stockdale S.R., Adriaenssens E.M., Kuhn J.H., Rumnieks J., Pallen M.J., Shkoporov A.N., Draper L.A., Ross R.P., Hill C. Leviviricetes: Expanding and Restructuring the Taxonomy of Bacteria-Infecting Single-Stranded RNA Viruses. Microb. Genom. 2021;7:686. doi: 10.1099/mgen.0.000686. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

