Dolutegravir-Based Regimen Ensures High Virological Success despite Prior Exposure to Efavirenz-Based First-LINE ART in Cameroon: An Evidence of a Successful Transition Model
- PMID: 36680058
- PMCID: PMC9866637
- DOI: 10.3390/v15010018
Dolutegravir-Based Regimen Ensures High Virological Success despite Prior Exposure to Efavirenz-Based First-LINE ART in Cameroon: An Evidence of a Successful Transition Model
Abstract
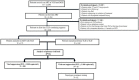
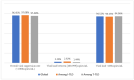
To ensure optimal prescribing practices in the dolutegravir-era in Cameroon, we compared first-line virological response (VR) under tenofovir + lamivudine + dolutegravir (TLD) according to prior exposure to tenofovir + lamivudine + efavirenz (TLE). A facility-based survey was conducted among patients initiating antiretroviral therapy (ART) with TLD (I-TLD) versus those transitioning from TLE to TLD (T-TLD). HIV viral load was performed and unsuppressed participants (VL > 1000 copies/mL) had genotyping performed by Sanger sequencing. Of the 12,093 patients followed, 310 (mean-age: 41 ± 11 years; 52.26% female) complied with study criteria (171 I-TLD vs. 139 T-TLD). The median ART-duration was 14 (12−17) months among I-TLDs versus 28 (24.5−31) months among T-TLDs (15 (11−19) on TLE and 14 (9−15) on TLD), and 83.15% (148/178) were at WHO clinical stages I/II. The viral suppression rate (<1000 copies/mL) was 96.45%, with 97.08% among I-TLDs versus 95.68% among T-TLDs (p = 0.55). VR was similar in I-TLD versus T-TLD at <400 copies/mL (94.15% versus 94.42%) and age, gender, residence, ART-duration, and WHO stages were not associated with VR (p > 0.05). Genotyping was successful for 72.7% (8/11), with no major mutations to integrase inhibitors found. VR is optimal under first-line TLD after 14 months, even among TLE-exposed, thus confirming the effectiveness of transitioning from TLE to TLD in similar settings, supported by strong pharmacological potency and genetic barrier of dolutegravir.
Keywords: Cameroon; HIV; TLD; antiretrovirals; first-line; virological response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Brief communication: Long-term treatment outcomes of transitioning to dolutegravir-based ART from efavirenz in HIV study participants in Mbeya, Tanzania.AIDS Res Ther. 2024 Dec 23;21(1):98. doi: 10.1186/s12981-024-00662-z. AIDS Res Ther. 2024. PMID: 39716209 Free PMC article.
-
Viral suppression and HIV-1 drug resistance 1 year after pragmatic transitioning to dolutegravir first-line therapy in Malawi: a prospective cohort study.Lancet HIV. 2022 Aug;9(8):e544-e553. doi: 10.1016/S2352-3018(22)00136-9. Lancet HIV. 2022. PMID: 35905753
-
Viral suppression in the era of transition to dolutegravir-based therapy in Cameroon: Children at high risk of virological failure due to the lowly transition in pediatrics.Medicine (Baltimore). 2023 May 19;102(20):e33737. doi: 10.1097/MD.0000000000033737. Medicine (Baltimore). 2023. PMID: 37335723 Free PMC article.
-
Abacavir/dolutegravir/lamivudine single-tablet regimen: a review of its use in HIV-1 infection.Drugs. 2015 Apr;75(5):503-14. doi: 10.1007/s40265-015-0361-6. Drugs. 2015. PMID: 25698454 Review.
-
Doing More With Less: Review of Dolutegravir-Lamivudine, a Novel Single-Tablet Regimen for Antiretroviral-Naïve Adults With HIV-1 Infection.Ann Pharmacother. 2020 Dec;54(12):1252-1259. doi: 10.1177/1060028020933772. Epub 2020 Jun 9. Ann Pharmacother. 2020. PMID: 32517480 Review.
Cited by
-
HIV susceptibility to second-generation integrase strand transfer inhibitors among people with multidrug resistance: a systematic review and meta-analysis protocol.Ther Adv Infect Dis. 2025 Apr 11;12:20499361251324914. doi: 10.1177/20499361251324914. eCollection 2025 Jan-Dec. Ther Adv Infect Dis. 2025. PMID: 40292085 Free PMC article.
-
Confirmed virological failure following enhanced adherence counseling among virally unsuppressed children and adolescents on dolutegravir-based versus other regimens: Evidence from a cohort analysis in Cameroon.Medicine (Baltimore). 2025 May 16;104(20):e42555. doi: 10.1097/MD.0000000000042555. Medicine (Baltimore). 2025. PMID: 40388726 Free PMC article.
-
Geospatial and temporal mapping of detectable HIV-1 viral loads amid dolutegravir rollout in KwaZulu-Natal, South Africa.PLOS Glob Public Health. 2024 May 28;4(5):e0003224. doi: 10.1371/journal.pgph.0003224. eCollection 2024. PLOS Glob Public Health. 2024. PMID: 38805477 Free PMC article.
-
Virology Outcomes of Tenofovir-lamivudine-dolutegravir in Treatment-naïve and Virologically Suppressed Individuals Switching From an NNRTI-based Regimen: An Observational Analysis at 13 Sites.Open Forum Infect Dis. 2025 May 2;12(7):ofaf270. doi: 10.1093/ofid/ofaf270. eCollection 2025 Jul. Open Forum Infect Dis. 2025. PMID: 40630936 Free PMC article.
-
HIV viral suppression in the era of dolutegravir use: Findings from a national survey in Tanzania.PLoS One. 2024 Aug 14;19(8):e0307003. doi: 10.1371/journal.pone.0307003. eCollection 2024. PLoS One. 2024. PMID: 39141647 Free PMC article.
References
-
- NIH-National Institute of Health Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. [(accessed on 28 May 2022)]; Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult....
-
- MINSANTE . Guide National De Prise En Charge Des Personnes Vivant Avec Le VIH/SIDA-Cameroun. Ministère de la Santé Publique; Yaoundé, Cameroun: 2012.
-
- MINSANTE . Plan National Multisectoriel De Lutte Contre Le Vih, Le Sida Et Les Ist (Pnm) Annee 2014–2017. Ministère de la Santé Publique; Yaoundé, Cameroun: 2013. pp. 1–163.
-
- MINSANTE . Directives Nationales De Prevention. Ministère de la Santé Publique; Yaoundé, Cameroun: 2014. p. 193.
-
- WHO . Lignes Directrices Unifiées Sur l’utilisation Des Antirétroviraux Pour Le Traitement et La Prévention De l’infection à VIH: Recommandations Pour Une Approche De Santé Publique. World Health Organization; Geneve, Switzerland: 2013. [(accessed on 2 March 2022)]. Available online: https://apps.who.int/iris/handle/10665/101196.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical