Analysis of Template Variations on RNA Synthesis by Respiratory Syncytial Virus Polymerase
- PMID: 36680087
- PMCID: PMC9863079
- DOI: 10.3390/v15010047
Analysis of Template Variations on RNA Synthesis by Respiratory Syncytial Virus Polymerase
Abstract
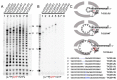
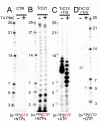
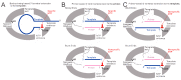
Respiratory syncytial virus (RSV) is a significant threat to infants and elderly individuals globally. Currently, there are no effective therapies or treatments for RSV infection because of an insufficient understanding of the RSV viral machinery. In this study, we investigated the effects of the template variations on RNA synthesis by the RSV polymerase through in vitro RNA synthesis assays. We confirmed the previously reported back-priming activity of the RSV polymerase, which is likely due to the secondary structure of the RNA template. We found that the expansion of the hairpin loop size of the RNA template abolishes the RSV polymerase back-priming activity. At the same time, it seemingly does not affect the de novo RNA synthesis activities of the RSV polymerase. Interestingly, our results show that the RSV polymerase also has a new primer-based terminal extension activity that adds nucleotides to the template and primer in a nonspecific manner. We also mapped the impact of the RNA 5' chemical group on its mobility in a urea-denaturing RNA gel shift assay. Overall, these results enhance our knowledge about the RNA synthesis processes of the RSV polymerase and may guide future therapeutic efforts to develop effective antiviral drugs for RSV treatment.
Keywords: RNA gel shift assay; RNA secondary structure; RNA-dependent RNA polymerase (RdRp); back-priming elongation; de novo RNA synthesis; primer-based elongation; respiratory syncytial virus (RSV); template variations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Knipe D.M., Howley P.M. Fields Virology. 6th ed. Volume 3 PA Wolters Kluwer/Lippincott Williams & Wilkins Health; Philadelphia, PA, USA: 2013.
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simões E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

