The Indirect ELISA and Monoclonal Antibody against African Swine Fever Virus p17 Revealed Efficient Detection and Application Prospects
- PMID: 36680090
- PMCID: PMC9865993
- DOI: 10.3390/v15010050
The Indirect ELISA and Monoclonal Antibody against African Swine Fever Virus p17 Revealed Efficient Detection and Application Prospects
Abstract
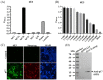
Since 2018, the outbreak and prevalence of the African swine fever virus (ASFV) in China have caused huge economic losses. Less virulent ASFVs emerged in 2020, which led to difficulties and challenges for early diagnosis and control of African swine fever (ASF) in China. An effective method of monitoring ASFV antibodies and specific antibodies against ASFV to promote the development of prevention techniques are urgently needed. In the present study, ASFV p17 was successfully expressed in CHO cells using a suspension culture system. An indirect enzyme-linked immunosorbent assay (ELISA) based on purified p17 was established and optimized. The monoclonal antibody (mAb) against p17 recognized a conservative linear epitope (3TETSPLLSH11) and exhibited specific reactivity, which was conducive to the identification of recombinant porcine reproductive and respiratory syndrome virus (PRRSV) expressing p17. The ELISA method efficiently detected clinical ASFV infection and effectively monitored the antibody levels in vivo after recombinant PRRSV live vector virus expressing p17 vaccination. Overall, the determination of the conserved linear epitope of p17 would contribute to the in-depth exploration of the biological function of the ASFV antigen protein. The indirect ELISA method and mAb against ASFV p17 revealed efficient detection and promising application prospects, making them ideal for epidemiological surveillance and vaccine research on ASF.
Keywords: ASFV p17; CHO cells; epitope; indirect ELISA; recombinant PRRSV.
Conflict of interest statement
The authors declare that they have no conflict of interest associated with this study.
Figures



Similar articles
-
A highly efficient indirect ELISA and monoclonal antibody established against African swine fever virus pK205R.Front Immunol. 2023 Jan 9;13:1103166. doi: 10.3389/fimmu.2022.1103166. eCollection 2022. Front Immunol. 2023. PMID: 36700212 Free PMC article.
-
Expression of ASFV p17 in CHO cells and identification of one novel epitope using a monoclonal antibody.Virus Res. 2023 Oct 15;336:199194. doi: 10.1016/j.virusres.2023.199194. Epub 2023 Aug 23. Virus Res. 2023. PMID: 37579847 Free PMC article.
-
Identification of a conservative site in the African swine fever virus p54 protein and its preliminary application in a serological assay.J Vet Sci. 2022 Jul;23(4):e55. doi: 10.4142/jvs.21134. Epub 2022 May 16. J Vet Sci. 2022. PMID: 35698809 Free PMC article.
-
Development of an ELISA Method to Differentiate Animals Infected with Wild-Type African Swine Fever Viruses and Attenuated HLJ/18-7GD Vaccine Candidate.Viruses. 2022 Aug 6;14(8):1731. doi: 10.3390/v14081731. Viruses. 2022. PMID: 36016353 Free PMC article.
-
African swine fever virus serodiagnosis: a general review with a focus on the analyses of African serum samples.Virus Res. 2013 Apr;173(1):159-67. doi: 10.1016/j.virusres.2012.10.021. Epub 2012 Nov 3. Virus Res. 2013. PMID: 23131491 Review.
Cited by
-
Identification of a New Conserved Antigenic Epitope by Specific Monoclonal Antibodies Targeting the African Swine Fever Virus Capsid Protein p17.Vet Sci. 2024 Dec 13;11(12):650. doi: 10.3390/vetsci11120650. Vet Sci. 2024. PMID: 39728990 Free PMC article.
-
Advancement in the Antigenic Epitopes and Vaccine Adjuvants of African Swine Fever Virus.Pathogens. 2024 Aug 21;13(8):706. doi: 10.3390/pathogens13080706. Pathogens. 2024. PMID: 39204306 Free PMC article. Review.
-
Establishment of an ELISA Based on a Recombinant Antigenic Protein Containing Multiple Prominent Epitopes for Detection of African Swine Fever Virus Antibodies.Microorganisms. 2024 May 7;12(5):943. doi: 10.3390/microorganisms12050943. Microorganisms. 2024. PMID: 38792774 Free PMC article.
-
Bridging the Gap: Can COVID-19 Research Help Combat African Swine Fever?Viruses. 2023 Sep 15;15(9):1925. doi: 10.3390/v15091925. Viruses. 2023. PMID: 37766331 Free PMC article. Review.
-
Establishment of an indirect ELISA antibody detection method based on the stable expression of LSDV P32 protein in CHO-K1 cells.BMC Biotechnol. 2025 Jun 4;25(1):45. doi: 10.1186/s12896-025-00966-6. BMC Biotechnol. 2025. PMID: 40468267 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

