Scratching the Surface Takes a Toll: Immune Recognition of Viral Proteins by Surface Toll-like Receptors
- PMID: 36680092
- PMCID: PMC9863796
- DOI: 10.3390/v15010052
Scratching the Surface Takes a Toll: Immune Recognition of Viral Proteins by Surface Toll-like Receptors
Abstract
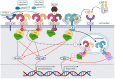
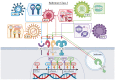
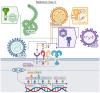
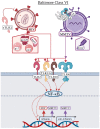
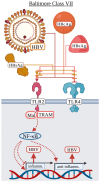
Early innate viral recognition by the host is critical for the rapid response and subsequent clearance of an infection. Innate immune cells patrol sites of infection to detect and respond to invading microorganisms including viruses. Surface Toll-like receptors (TLRs) are a group of pattern recognition receptors (PRRs) that can be activated by viruses even before the host cell becomes infected. However, the early activation of surface TLRs by viruses can lead to viral clearance by the host or promote pathogenesis. Thus, a plethora of research has attempted to identify specific viral ligands that bind to surface TLRs and mediate progression of viral infection. Herein, we will discuss the past two decades of research that have identified specific viral proteins recognized by cell surface-associated TLRs, how these viral proteins and host surface TLR interactions affect the host inflammatory response and outcome of infection, and address why controversy remains regarding host surface TLR recognition of viral proteins.
Keywords: TLR agonist; TLR antagonist; TLR2; TLR4; Toll-like receptors; cytokines; innate immunity; interferons; viral proteins; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

